Figure 3 |. Identification of an extrahelical lipid-facing allosteric binding pocket involving TMs 1,6 & 7 on the A1R.
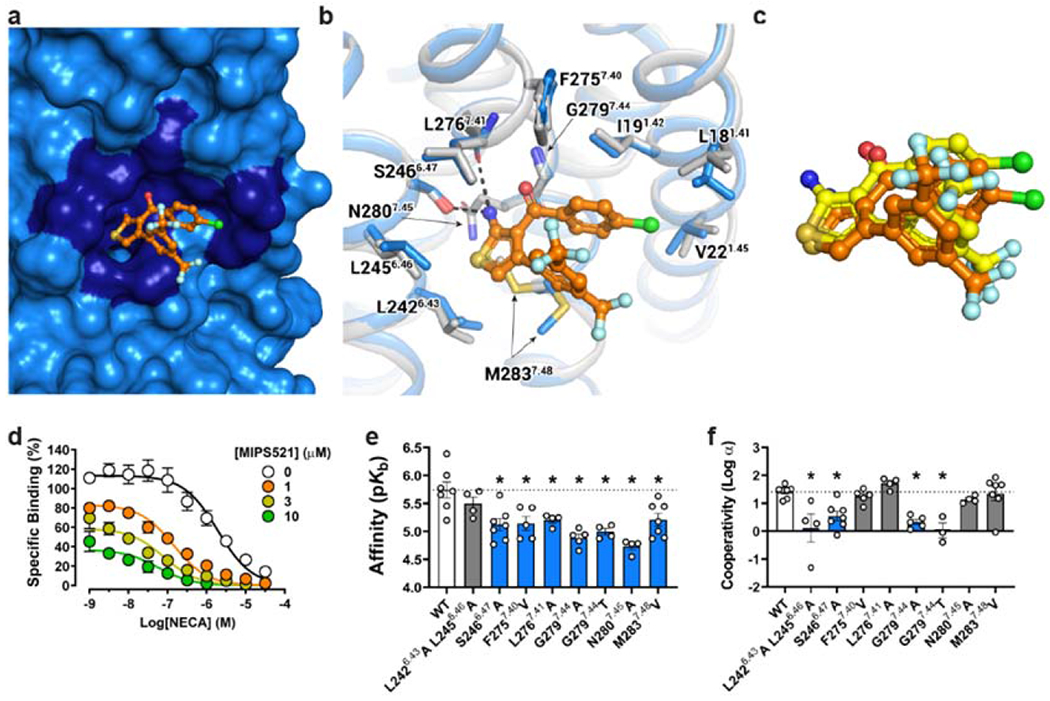
a, Surface rendering of the MIPS521 binding site located in an extrahelical position between TM6 & 7. b, Extrahelical allosteric binding pocket of the A1R in the presence and absence of MIPS521, with a pivoting of M2837.48 to accommodate MIPS521 (Pocket residues in stick representation coloured by heteroatom; MIPS521-ADO-A1R-Gi2 complex (blue), MIPS521 (orange); ADO-A1R-Gi2 complex (grey), c, Comparison of the binding pose of MIPS521 from the cryo-EM structure (orange) and in situ docking (yellow), d, Allosteric modulation of orthosteric ligand affinity at the WT A1R demonstrated in a [3H]-DPCPX radioligand interaction binding assay in the presence of the orthosteric agonist NECA and MIPS521. Data are presented as mean +/− SEM, n=6. e, f, Changes in MIPS521 affinity (pKB) (e) or binding cooperativity (log a) with the orthosteric agonist NECA (f) following mutation of residues proposed to form the allosteric pocket identified in the cryo-EM structure. Parameter estimates are the mean ± SEM determined from n=3-6 (white circles) performed in duplicate. *P < 0.05 (compared with wild type; one-way ANOVA, Dunnett’s post hoc test).
