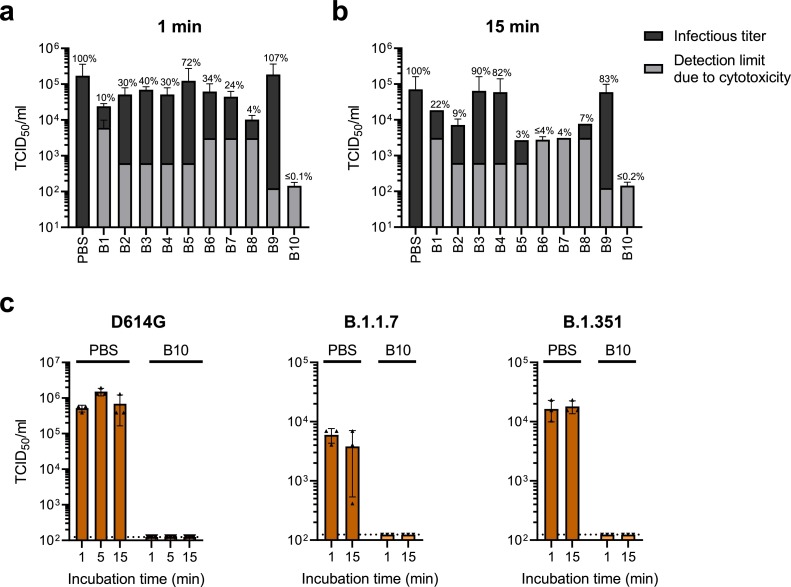
Fig. 1.
Virucidal activity of extraction buffers in rapid SARS-CoV-2 antigen tests. a, b) A solution of 10% SARS-CoV-2 D614G, 10% interfering substance (5% (w/v) albumin fraction V, 0.4% (w/v) mucin from bovine submaxillary glands type I-S, 5% (w/v) yeast extract) and 80% of the respective buffer B1-B10 or phosphate buffer saline (PBS) was analyzed according to quantitative suspension tests as described in DIN EN 14,476 [8,9]. After incubation for (a) 1 min or (b) 15 min, viral infectivity was quantified by TCID50 endpoint titration on Vero E6 cells. Detection limits caused by cytotoxicity of buffers are shown in gray, infectious titers in black. c) 10% virus (D614G variant or VOCs B.1.1.7 or B.1.351) was spiked into 90% pooled saliva and mixed 1:4 with B10 or PBS. After the indicated incubation times, an endpoint titration on Vero E6 cells was performed to determine TCID50/ml. Dotted lines indicate limit of detection due to cytotoxicity. a–c) Shown are means of three independent experiments ± SD.