Objectives:
Long-acting formulations of cabotegravir (CAB) and rilpivirine (RPV) have demonstrated efficacy in Phase 3 studies. POLAR (NCT03639311) assessed antiviral activity and safety of CAB+RPV long-acting administered every 2 months (Q2M) in adults living with HIV-1 who previously received daily oral CAB+RPV in LATTE (NCT01641809).
Design:
A Phase 2b, multicenter, open-label, rollover study.
Methods:
LATTE participants with plasma HIV-1 RNA less than 50 copies/ml who completed at least 300 weeks on study were eligible. Participants elected to switch to either CAB+RPV long-acting Q2M or daily oral dolutegravir/RPV for maintenance of virologic suppression. The primary endpoint was the proportion of participants with HIV-1 RNA greater than or equal to 50 copies/ml at Month 12 (M12) per the Food and Drug Administration Snapshot algorithm. The incidence of confirmed virologic failure (CVF, two consecutive HIV-1 RNA measurements greater than or equal to 200 copies/ml), as well as safety, laboratory, and patient-reported outcomes (HIV Treatment Satisfaction and preference questionnaires) were also assessed.
Results:
Of 97 participants enrolled, 90 chose to receive CAB+RPV long-acting and seven chose dolutegravir/RPV. At M12, no participant had HIV-1 RNA greater than or equal to 50 copies/ml or met the CVF criterion in either treatment group. No new safety signals were identified. Total treatment satisfaction was high at Baseline and remained stable through M12 across both treatment groups. Overall, 88% (n = 77/88) of long-acting arm participants preferred CAB+RPV long-acting to oral CAB+RPV.
Conclusion:
CAB+RPV long-acting maintained virologic suppression in participants who had previously received daily oral CAB+RPV for at least 5 years in LATTE, with a favorable safety profile. Most participants preferred CAB+RPV long-acting to their prior oral CAB+RPV regimen at M12.
Keywords: cabotegravir, HIV-1, injectable, intramuscular, long-acting, maintenance, rilpivirine
Introduction
Advances in the development of new antiretroviral therapies (ARTs) have substantially improved treatments for people with HIV (PWH) [1]. However, several patient-related challenges have been linked to the current treatment paradigm of lifelong adherence to daily oral pill taking. These include the maintenance of such adherence, taking personal day-to-day responsibility for care, stigmatization concerns, and the daily reminder of HIV status, as well as intrinsic medical issues, such as difficulty swallowing and drug–food and drug–drug interactions [2–6]. These challenges can impact the likelihood of treatment failure, as even small digressions in adherence to daily oral ART are believed to increase the risk of treatment-emergent resistance, contributing to virologic rebound [7–12]. Treatment adherence is critical to achieve the Joint United Nations Programme on HIV/AIDS (UNAIDS) 95–95–95 2030 target, an ambitious goal set to ensure that 95% of all individuals receiving ART will achieve and sustain viral suppression [13]. Consequently, there is a need to develop new ARTs that offer more convenient dosing while retaining high rates of virologic suppression, an acceptable safety profile, and a high barrier to resistance. Injectable long-acting ARTs have the potential to mitigate some of the challenges associated with daily oral regimens, offering reduced dosing frequencies and bypassing the gastrointestinal tract to avoid many common drug–food and drug–drug interactions. In addition, long-acting regimens have the potential to improve adherence by minimizing the impacts of forgetfulness and high pill burdens [4].
Cabotegravir (CAB), an integrase strand transfer inhibitor, and rilpivirine (RPV), a nonnucleoside reverse transcriptase inhibitor, are two agents for which an approved complete monthly (Australia, USA, Canada, and EU) and every 2 month (Q2M; Australia, Canada, and EU) long-acting injectable dosing regimen has been developed [14–16]. CAB+RPV long-acting is indicated for the treatment of HIV-1 infection in virologically suppressed adults (HIV-1 RNA < 50 copies/ml) [14–16]. Regulatory approval was based on several large clinical studies, including two pivotal randomized Phase 3 studies, ATLAS (NCT02951052) and FLAIR (NCT02938520), which demonstrated the noninferiority of intramuscular (i.m.) injections of CAB+RPV long-acting dosed every 4 weeks as a maintenance therapy compared with daily oral comparator regimens [17,18]. A reduced dosing frequency for CAB+RPV long-acting of every 8 weeks was shown to be noninferior to every 4 week dosing in the Phase 3b ATLAS-2 M study (NCT03299049) [19]. The Phase 2b LATTE study (NCT01641809) preceded the ATLAS, FLAIR, and ATLAS-2 M long-acting clinical evaluations, investigating daily oral formulations of CAB+RPV compared with three-drug efavirenz-based ART for the maintenance of viral suppression [20]. LATTE demonstrated the antiviral activity of the two-drug oral maintenance therapy CAB+RPV was comparable to efavirenz plus dual nucleoside reverse transcriptase inhibitors at Week 96. POLAR (NCT03639311) is a Phase 2b rollover study assessing the antiviral activity and safety of CAB+RPV long-acting Q2M in ART-experienced participants who received once-daily oral CAB+RPV treatment for at least 5 years in the Phase 2b LATTE study. The Month 12 results from POLAR are presented here.
Materials and methods
Study design and participants
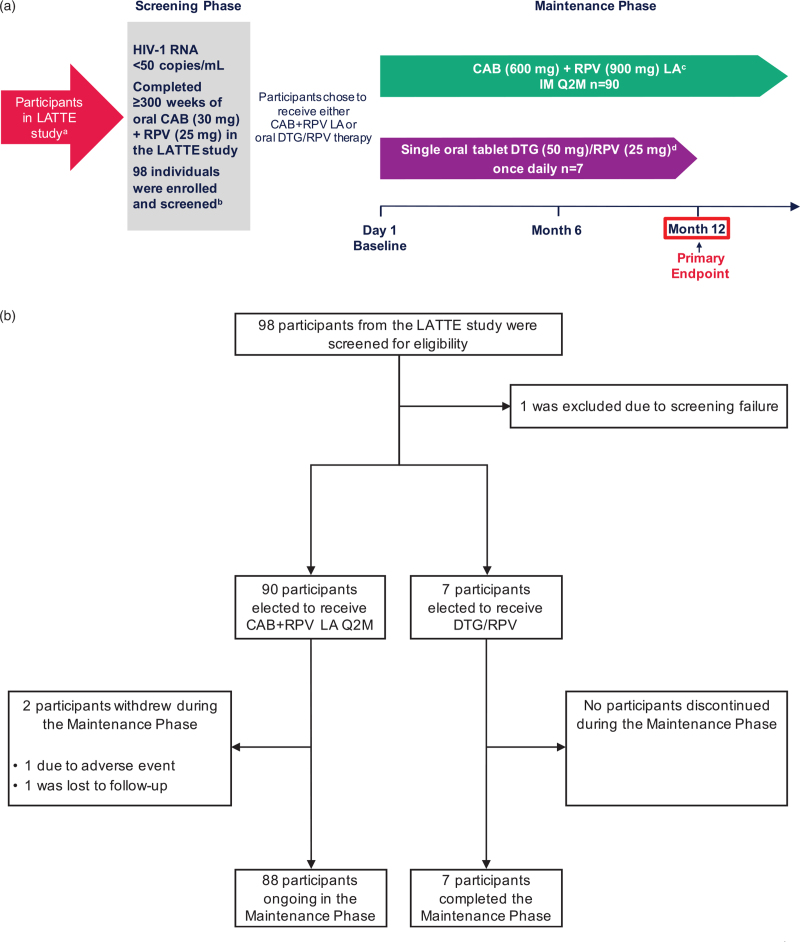
POLAR is a Phase 2b, open-label, multicenter (Canada and the USA), nonrandomized rollover study assessing the efficacy and safety of i.m. CAB+RPV long-acting Q2M in ART-experienced adults living with HIV-1 who received once-daily oral CAB+RPV treatment in the Phase 2b LATTE study (Fig. 1a). The full study protocol is available at ClinicalTrials.gov: NCT03639311. In brief, eligible participants were at least 18 years of age, virologically suppressed, and had completed at least 300 weeks of the LATTE study with plasma HIV-1 RNA less than 50 copies/ml at Week 300. If participants had plasma HIV-1 RNA greater than or equal to 50 copies/ml at Week 300, a single repeat test to determine eligibility was allowed after consultation with the medical monitor. Participants from LATTE were excluded if they had two or more sequential plasma HIV-1 RNA greater than or equal to 50 copies/ml or any plasma HIV-1 RNA greater than or equal to 200 copies/ml in the last 6 months.
Fig. 1.
Study design, screening, and treatment.
aParticipants in LATTE received daily oral CAB (30 mg) + RPV (25 mg). bOne individual failed screening with the primary reason being “not meeting the inclusion or exclusion criteria.” cParticipants received CAB LA 600 mg + RPV LA 900 mg at Day 1 and Month 2, then Q2M thereafter. To be accessed commercially once CAB+RPV LA Q2M is approved. Any participant who received at least one dose of CAB LA and/or RPV LA and discontinued the CAB+RPV LA regimen for any reason entered a 52-week Long-Term Follow-Up Phase and transitioned to an alternative ART regimen. dTo be accessed longer term via a commercial route. Participants will continue to receive DTG/RPV if located in a region where not commercially available. Figure 1a was presented previously at IDWeek; October 21–25, 2020; Virtual; Oral. ART, antiretroviral therapy; CAB, cabotegravir; DTG, dolutegravir; IM, intramuscular; LA, long-acting; Q2M, every 2 months; RPV, rilpivirine.
The study included a Maintenance Phase (Day 1 until CAB+RPV long-acting is commercially available locally) and a Long-Term Follow-Up Phase (LTFU, 52 weeks) for participants who discontinued for any reason after receiving at least one dose of CAB long-acting and/or RPV long-acting. Participants entered the study on Day 1 and elected to transition from daily oral CAB+RPV to either i.m. CAB+RPV long-acting Q2M (long-acting arm; CAB long-acting 600 mg and RPV long-acting 900 mg) or daily oral single-tablet dolutegravir (DTG) 50 mg/RPV 25 mg (DTG/RPV arm) for 12 months. To cover preplanned, short-term interruptions of long-acting dosing, investigators could provide daily oral CAB 30 mg and RPV 25 mg as an oral therapy following consultation with the medical monitor. Participants in the long-acting arm will continue to receive CAB+RPV long-acting Q2M until the regimen is approved and commercially available within the local sector (including through local public/government health sectors), the participant no longer derives clinical benefit, or the participant meets a protocol-defined reason for discontinuation. DTG/RPV tablets were provided via commercial routes. Any participant who received at least one dose of CAB long-acting and/or RPV long-acting and discontinued CAB+RPV long-acting for any reason entered a 52-week LTFU Phase and remained on an alternative suppressive ART regimen for at least 52 weeks after the last dose. The cut-off date for this analysis predates the COVID-19 2020 pandemic. POLAR was conducted in accordance with the principles founded in the Declaration of Helsinki and with Good Clinical Practice [21,22]. All participants provided written informed consent, and the protocol was approved by an institutional review board or ethics committee.
Endpoints and assessments
The primary objective of the study was to demonstrate the antiviral activity of CAB+RPV long-acting Q2M in suppressed HIV-1-infected ART-experienced participants. The primary endpoint was the proportion of participants with HIV-1 RNA greater than or equal to 50 copies/ml at Month 12 as per the Food and Drug Administration (FDA) Snapshot algorithm. Secondary endpoints assessed at Month 12 included the proportion of participants with plasma HIV-1 RNA less than 50 copies/ml at Month 12 as per the FDA Snapshot algorithm; the incidence of protocol-defined confirmed virologic failure (CVF; two consecutive plasma HIV-1 RNA measurements of ≥200 copies/ml); incidence of treatment-emergent genotypic and phenotypic resistance; absolute values and changes in CD4+ cell count over time; incidence and severity of adverse events and laboratory abnormalities; proportion of participants who discontinue treatment due to adverse events; change from Baseline in laboratory parameters over time; and treatment satisfaction, as measured by the 12-item HIV Treatment Satisfaction Questionnaire status (HIVTSQs) and change (HIVTSQc) versions at Baseline, Month 6, and Month 12. HIVTSQ responses are rated on 6-point Likert scales, with the HIVTSQs total score ranging from 0 (very dissatisfied) to 66 (very satisfied), and the HIVTSQc total score ranging from –33 (much less satisfied now) to 33 (much more satisfied now). An exploratory endpoint evaluating participant preference for CAB+RPV long-acting compared with their prior daily oral CAB+RPV regimen was also included (single-item questionnaire) at Month 12. The pharmacokinetic profiles of CAB and RPV were not investigated in the study as they have been extensively characterized in previous Phase 2 and Phase 3 studies of the daily oral regimen and the i.m. therapy given every 4 or 8 weeks [17–19,23].
Statistical analysis
No statistical hypotheses of treatment comparisons were tested within this study due to the nonrandomized nature of the study and the unbalanced number of participants between comparator arms. Consequently, all presented data are descriptive.
Results
Participants
Of the 98 participants from the LATTE study who were screened for eligibility, 97 entered the Maintenance Phase of POLAR [intention-to-treat exposed (ITT-E) population]. One participant failed screening due to abnormal electrocardiogram results (prolonged QTc interval). Participant disposition is shown in Fig. 1b. Of those enrolled, 90 of 97 participants (93%) elected to receive CAB+RPV long-acting therapy, while the remaining seven of 97 (7%) chose to receive daily oral DTG/RPV. Baseline participant characteristics are summarized in Table 1.
Table 1.
Baseline participant characteristics.
| Parameter | IM CAB+RPV LA Q2M arm n = 90 | Oral DTG/RPV QD arm n = 7 | Total N = 97 |
| Age, median (range) years | 41 (25–63) | 53 (30–62) | 41 (25–63) |
| Age ≥50 years, n (%) | 16 (18) | 4 (57) | 20 (21) |
| Female (sex at birth), n (%) | 2 (2) | 0 | 2 (2) |
| Female (self-reported sex), n (%) | 3 (3) | 0 | 3 (3) |
| Race, n (%) | |||
| White | 63 (70) | 4 (57) | 67 (69) |
| Black or African–American | 21 (23) | 3 (43) | 24 (25) |
| Other | 6 (7) | 0 | 6 (6) |
| BMI, median (range) kg/m2 | 27 (19–48) | 27 (24–31) | 27 (19–48) |
| CD4+ cell count, median (range) cells/μl | 851 (376–1593) | 779 (595–1050) | 842 (376–1593) |
Table 1 was presented previously at IDWeek; October 21–25, 2020; Virtual; Oral.
CAB, cabotegravir; DTG, dolutegravir; IM, intramuscular; LA, long-acting; QD, once daily; Q2M, every 2 months; RPV, rilpivirine.
Efficacy
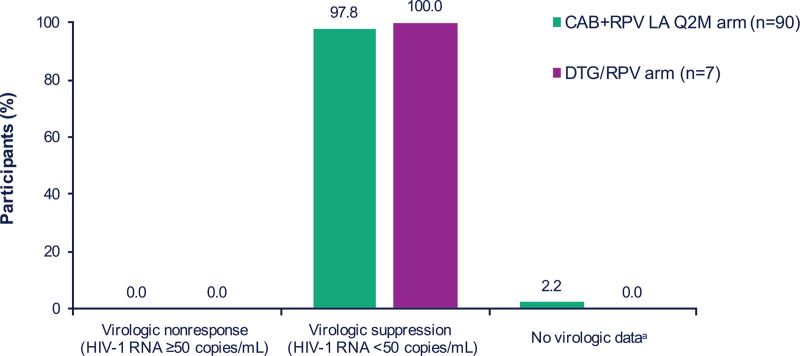
In the ITT-E population, no participant in either treatment arm had HIV-1 RNA greater than or equal to 50 copies/ml at Month 12, as per the FDA Snapshot algorithm (Fig. 2 and Supplemental Digital Content, Table S1, Efficacy outcomes at Month 12). Overall, 88 of 90 (98%) and seven of seven (100%) participants in the CAB+RPV long-acting and DTG/RPV arms, respectively, maintained virologic suppression at Month 12. The remaining two (2%) participants in the CAB+RPV long-acting arm had no virologic data at Month 12; one discontinued treatment due to an adverse event and the other was lost to follow-up. Through Month 12, no participants met the CVF criterion in either treatment arm. Consequently, no resistance data were generated. At Month 12, following at least 6 years of CAB+RPV therapy (including ∼300 weeks of oral CAB+RPV in LATTE), the median [interquartile range (IQR)] CD4+ cell count change from Baseline in POLAR was –12.5 (–138.0, 71.0) cells/μl in the CAB+RPV long-acting arm and –68.0 (–152.0, 152.0) cells/μl in the DTG/RPV arm. CD4+ cell counts prior to initiating oral CAB+RPV are detailed in the primary publication of the LATTE study [20].
Fig. 2.
Efficacy outcomes at Month 12.
aTwo (2%) participants in the LA arm had no virologic data at Month 12; one discontinued treatment due to an AE and the other was lost to follow-up. Figure 2 was presented previously at IDWeek; October 21–25, 2020; Virtual; Oral. AE, adverse event; CAB, cabotegravir; DTG, dolutegravir; LA, long-acting; Q2M, every 2 months; RPV, rilpivirine.
Safety and tolerability
Through Month 12, 86 of 90 (96%) participants in the CAB+RPV long-acting arm and three of seven (43%) in the DTG/RPV arm reported adverse events (Table 2). All reported drug-related adverse events were of mild (Grade 1) or moderate (Grade 2) severity, most of which were injection site reactions (ISRs) in the CAB+RPV long-acting arm. The most frequently reported non-ISR drug-related adverse events in the CAB+RPV long-acting arm were pyrexia (8%, n = 7/90) and fatigue (4%, n = 4/90). One participant experienced a drug-related adverse event in the DTG/RPV arm (headache). There was one withdrawal due to an adverse event (CAB+RPV long-acting arm; drug-related adverse event of depression) and one drug-related serious adverse event (CAB+RPV long-acting arm; injection site extravasation) that resolved by drainage on the same day and did not lead to study discontinuation. No clinically relevant patterns of adverse events over the 12-month period were observed.
Table 2.
Adverse event overview.
| Parameter, n (%) | IM CAB+RPV LA Q2M arm n = 90 | Oral DTG/RPV QD arm n = 7 |
| Any AE | 86 (96) | 3 (43) |
| Grade ≥3 AE | 9 (10) | 0 |
| Common AEs (≥5% in either arm, excluding ISRs), n (%) | ||
| Nasopharyngitis | 10 (11) | 0 |
| Upper respiratory tract infection | 10 (11) | 0 |
| Diarrhea | 9 (10) | 0 |
| Pyrexia | 9 (10) | 0 |
| Headache | 6 (7) | 1 (14) |
| Fatigue | 6 (7) | 0 |
| Syphilis | 6 (7) | 0 |
| Cough | 5 (6) | 0 |
| Hemorrhoids | 5 (6) | 0 |
| Nausea | 5 (6) | 0 |
| Abdominal pain | 2 (2) | 1 (14) |
| Muscle strain | 1 (1) | 1 (14) |
| Erythema | 0 | 1 (14) |
| Hepatic steatosis | 0 | 1 (14) |
| Drug-related AEs | 65 (72) | 1 (14) |
| Grade ≥3 AE | 0 | 0 |
| Common drug-related AEs (≥3% in either arm, excluding ISRs), n (%) | ||
| Pyrexia | 7 (8) | 0 |
| Fatigue | 4 (4) | 0 |
| Pain | 3 (3) | 0 |
| Headache | 2 (2) | 1 (14) |
| AEs leading to withdrawal | 1 (1)a | 0 |
| Drug-related AEs leading to withdrawal | 1 (1)a | 0 |
| Any SAE | 5 (6)b | 0 |
| Drug-related SAEs | 1 (1)c | 0 |
Drug-related AE of depression.
SAEs included cholecystitis acute (n = 1), cholelithiasis (n = 1), anal abscess (n = 1), orchitis (n = 1), urinary tract infection bacterial (n = 1), proctitis (n = 1), and injection site extravasation (n = 1). Participants could have experienced >1 SAE.
Drug-related SAE of injection site extravasation.
Table 2 was presented previously at IDWeek; October 21–25, 2020; Virtual; Oral.
AE, adverse event; CAB, cabotegravir; DTG, dolutegravir; IM, intramuscular; ISR, injection site reaction; LA, long-acting; QD, once daily; Q2M, every 2 months; RPV, rilpivirine; SAE, serious adverse event.
Injection site reactions
In total, 70 of 90 (78%) participants in the CAB+RPV long-acting arm reported at least one ISR, for a total of 463 ISR events (Supplemental Digital Content, Table S2, ISR overview). All ISR events were either mild (Grade 1; 84%, n = 389/463) or moderate (Grade 2; 16%, n = 74/463) in severity, and most (92%, n = 424/463) resolved within 7 days, with a median (IQR) duration of 3 (2, 4) days. The incidence of ISRs decreased over the study period (Supplemental Digital Content, Figure S1, ISR incidence over time through Month 12), with 67% (n = 60/90) of participants reporting ISRs on Day 1 compared with 26% (n = 23/88) at the Month 12 visit. The most commonly reported ISRs in the CAB+RPV long-acting arm (≥5% of participants) were injection site pain (74%, n = 67), injection site discomfort (11%, n = 10), injection site swelling (6%, n = 5), and injection site nodule (6%, n = 5). No participants withdrew from the study due to an ISR or injection intolerability.
Weight change
At Baseline, the median (range) weight was 85.6 kg (56.3, 177.3 kg) in the CAB+RPV long-acting arm and 82.0 kg (65.0, 98.3 kg) in the DTG/RPV arm. At Month 12, there was a median weight increase of 1.6 kg (–4.5, 10.5 kg) from Baseline in the DTG/RPV arm, while no change from Baseline in median weight was observed in the CAB+RPV long-acting arm (–22.2, 16.8 kg). A decrease in weight was classified as an adverse event for one participant in the CAB+RPV long-acting arm but was deemed unrelated to treatment.
Patient-reported outcomes
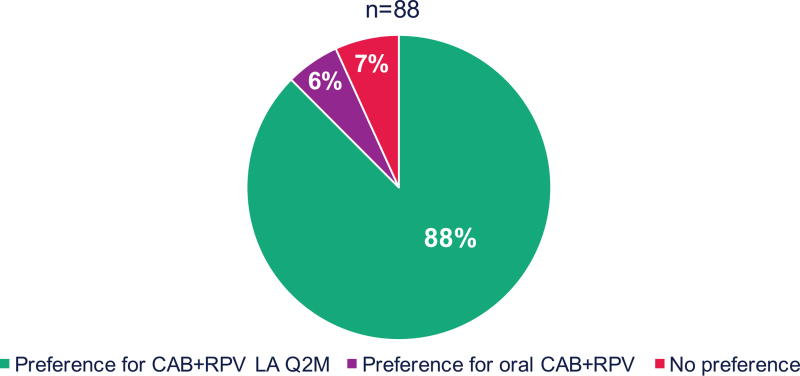
HIVTSQs mean [standard deviation (SD)] total scores were high at Baseline [CAB+RPV long-acting arm, 62.83 (4.88); DTG/RPV, 63.71 (3.68)] and remained stable at Months 6 [CAB+RPV long-acting arm, 62.60 (5.61); DTG/RPV, 64.43 (3.05)] and 12 [CAB+RPV long-acting arm, 62.38 (6.46); DTG/RPV, 63.86 (4.49)] across both treatment groups. At Month 12, mean (SD) total HIVTSQc scores, representing changes in satisfaction from prior therapy, were high in both treatment groups [CAB+RPV long-acting arm, 28.0 (6.78); DTG/RPV arm, 27.7 (6.97)], with positive mean change scores observed for all individual items for both arms (Supplemental Digital Content, Figure S2, Mean HIVTSQc individual item scores at Month 12). An exploratory analysis of therapy preference at Month 12 indicated that, of the 88 participants in the CAB+RPV long-acting arm with a recorded response to the preference questionnaire, 88% (n = 77/88) preferred CAB+RPV long-acting Q2M over daily oral CAB+RPV, the regimen received in the LATTE study for at least 5 years (Fig. 3). The most commonly cited reasons for preference among the 83 participants who provided responses included increased convenience (69%, n = 57/83) and the frequency of administration (57%, n = 47/83).
Fig. 3.
Treatment preference at Month 12 for participants receiving CAB+RPV LA Q2M.
Figure 3 was presented previously at IDWeek; October 21–25, 2020; Virtual; Oral. CAB, cabotegravir; LA, long-acting; Q2M, every 2 months; RPV, rilpivirine.
Discussion
The results from the POLAR study complement those collected from larger Phase 3 studies and together support the therapeutic potential of i.m. CAB+RPV long-acting administered monthly or Q2M for the maintenance of virologic suppression in PWH [17–19]. Notably, the POLAR study is the first clinical study to investigate the CAB+RPV long-acting regimen dosed Q2M, as indicated, instead of every 8 weeks.
The high level of virologic suppression and low rate of CVF reflect the results obtained from the larger Phase 3 studies of CAB+RPV long-acting, and contribute to a growing library of clinical evidence supporting its efficacy as a maintenance regimen [17–19]. Furthermore, the safety and tolerability profile was consistent with previous studies and no new safety signals were identified [17–19]. As previously observed, ISRs were of mild-to-moderate intensity, of short duration, and decreased in incidence over time [17–19]. Notably, ISRs did not lead to treatment discontinuation.
Participants transitioning from the Phase 2b LATTE study were treatment experienced, having received daily oral CAB+RPV for at least 5 years. At the beginning of the Maintenance Phase, 93% (n = 90/97) of study participants elected to receive CAB+RPV long-acting over daily oral tablets. This may reflect the substantial interest in long-acting therapies observed in the population of PWH and serves as an encouraging sign for the clinical uptake of the injectable regimen [24–28]. Notably, participants were more satisfied with the regimens that they chose to receive in POLAR (both CAB+RPV long-acting and oral DTG/RPV) than with the daily oral CAB+RPV regimen they had previously received for at least 5 years, as measured by the HIVTSQc. In addition, 88% (n = 77/88) of CAB+RPV long-acting respondents stated a preference for CAB+RPV long-acting over their prior oral regimen, consistent with findings from the other Phase 3 studies (ATLAS, FLAIR, and ATLAS-2 M) evaluating CAB+RPV long-acting [17–19,29–31]. Taken together, these results demonstrate high levels of preference for, and satisfaction with, the injectable regimen.
Limitations
Owing to the small number of participants enrolled, the study was not adequately powered to draw statistical inferences from the results. Further, as participants could choose which maintenance regimen they received, POLAR was nonrandomized and, therefore, the numbers of participants differed greatly between comparator arms. As a result, comparisons between CAB+RPV long-acting and DTG/RPV cannot be drawn and any differences in results must be interpreted within this context. In addition, all participants had completed more than 300 weeks of oral CAB+RPV therapy prior to study entry and are therefore a highly selected population comprising participants with established efficacy with, and tolerability to, CAB+RPV. This caveat limits the generalizability of the findings to a wider population initiating CAB+RPV long-acting as a novel regimen. The small number of females (sex at birth) in the study should also be considered in the interpretation of the findings. The design of the study prevented blinding, which could influence the reporting of adverse events and patient-reported outcomes; however, this would not affect the efficacy findings, which were based on objective measurements of viral load.
Conclusion
Injectable CAB+RPV long-acting, administered Q2M, maintained high levels of virologic suppression in participants previously treated with oral CAB+RPV, with no participants having virologic nonresponse or meeting the CVF criterion through Month 12 of the POLAR study. The injectable CAB+RPV long-acting regimen was well tolerated and preferred over oral therapy by this treatment-experienced cohort, who had previously received daily oral CAB+RPV for at least 5 years in the LATTE study. Taken together, these results complement the results observed in larger Phase 3 studies evaluating the injectable regimen and support CAB+RPV long-acting as an efficacious and well-tolerated Q2M maintenance therapy for PWH.
Acknowledgements
The authors thank everyone who has contributed to the success of the study: all study participants and their families, and the clinical investigators and their staff. Professional medical writing and editorial assistance was provided by Euan Paul at SciMentum (Nucleus Global) and funded by ViiV Healthcare.
The POLAR study (NCT03639311) was funded by ViiV Healthcare and Janssen Pharmaceuticals.
A.M., G.J.R., C.N., O.O., J.C., C.B., and J.D.V. participated in the collection and interpretation of the data. D.A.M., K.C.S., V.W., S.H., J.R., C.M., C.G., K.V., and W.R.S. were responsible for the development, conception, and data analysis of the study. All authors contributed to the review and development of the manuscript.
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com
Conflicts of interest
A.M. has received research funding and consulting fees from ViiV Healthcare, Gilead, Janssen Pharmaceuticals, and Merck, and consulting fees from Shionogi & Co., Ltd. G.J.R. reports grants from Gilead, ViiV Healthcare, and TaiMed. C.N. reports consulting fees from GlaxoSmithKline and ViiV Healthcare, and research support from GlaxoSmithKline and Gilead. O.O. received consulting fees from Gilead, ViiV Healthcare, and Merck. J.C. reports grants, consulting fees, and speaker fees from Gilead, ViiV Healthcare, and Merck. C.B. has received speaker fees from ViiV Healthcare and Gilead and other from GlaxoSmithKline, Janssen Pharmaceuticals, and Sangamo, outside the submitted work.
J.D.V. has nothing to disclose. D.A.M. was an employee of ViiV Healthcare and stockholder of GlaxoSmithKline. K.C.S., C.M., C.G., and W.R.S. are employees of ViiV Healthcare and stockholders of Glaxo-SmithKline. V.W., S.H., and J.R. are employees and stockholders of GlaxoSmithKline. K.V. is an employee and stockholder of Janssen Pharmaceuticals.
Previous presentation: IDWeek; October 21–25, 2020; Virtual; Oral.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Freedberg KA, Sax PE. Improving on effective antiretroviral therapy: how good will a cure have to be?. J Med Ethics 2017; 43:71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, et al. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc 2013; 16: (3 Suppl 2): 18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iacob SA, Iacob DG, Jugulete G. Improving the adherence to antiretroviral therapy, a difficult but essential task for a successful HIV treatment-clinical points of view and practical considerations. Front Pharmacol 2017; 8:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shubber Z, Mills EJ, Nachega JB, Vreeman R, Freitas M, Bock P, et al. Patient-reported barriers to adherence to antiretroviral therapy: a systematic review and meta-analysis. PLOS Med 2016; 13:e1002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swindells S, Flexner C, Fletcher CV, Jacobson JM. The critical need for alternative antiretroviral formulations, and obstacles to their development. J Infect Dis 2011; 204:669–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Los Rios P, Young B, Marcotullio S, Punekar Y, Koteff J, Ustianowski A, et al. 1329. Experiences and emotional challenges of antiretroviral treatment (ART)—findings from the Positive Perspectives Study. Open Forum Infect Dis 2019; 6: (Suppl 2): S481. [Google Scholar]
- 7.Bangsberg DR, Perry S, Charlebois ED, Clark RA, Roberston M, Zolopa AR, et al. Nonadherence to highly active antiretroviral therapy predicts progression to AIDS. AIDS 2001; 15:1181–1183. [DOI] [PubMed] [Google Scholar]
- 8.Biset Ayalew M. Mortality and its predictors among HIV infected patients taking antiretroviral treatment in Ethiopia: a systematic review. AIDS Res Treat 2017; 2017:5415298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai S, Mahapatra B, Sircar S, Raj PY, Venkatesh S, Shaukat M, et al. Adherence to antiretroviral therapy and its effect on survival of HIV-infected individuals in Jharkhand, India. PLoS One 2013; 8:e66860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachega JB, Marconi VC, van Zyl GU, Gardner EM, Preiser W, Hong SY, et al. HIV treatment adherence, drug resistance, virologic failure: evolving concepts. Infect Disord Drug Targets 2011; 11:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li JZ, Paredes R, Ribaudo HJ, Svarovskaia ES, Metzner KJ, Kozal MJ, et al. Low-frequency HIV-1 drug resistance mutations and risk of NNRTI-based antiretroviral treatment failure: a systematic review and pooled analysis. JAMA 2011; 305:1327–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meshesha HM, Nigussie ZM, Asrat A, Mulatu K. Determinants of virological failure among adults on first-line highly active antiretroviral therapy at public health facilities in Kombolcha town, Northeast, Ethiopia: a case–control study. BMJ Open 2020; 10:e036223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. UNAIDS. Understanding fast-track: accelerating action to end the AIDS epidemic by 2030. 2015. Available at https://www.unaids.org/sites/default/files/media_asset/201506_JC2743_Understanding_FastTrack_en.pdf. [Accessed 23 February 2021] [Google Scholar]
- 14. ViiV Healthcare. Vocabria summary of product characteristics. EU, January 2021. Available at https://www.ema.europa.eu/en/documents/product-information/vocabria-epar-product-information_en.pdf [Accessed 1 October 2021] [Google Scholar]
- 15. ViiV Healthcare. Vocabria (cabotegravir tablets) and Cabenuva (cabotegravir and rilpivirine extended release injectable suspensions) Product Monograph. Canada, March 2020. Available at https://viivhealthcare.com/content/dam/cf-viiv/viiv-healthcare/en_GB/medicines/CABENUVA-VOCABRIA-PM-26-Mar-2021.pdf [Accessed 1 October 2021] [Google Scholar]
- 16. ViiV Healthcare. Cabotegravir extended-release injectable suspension; rilpivirine extended-release injectable suspension (Cabenuva) Prescribing Information. US, January 2021. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/212888s000lbl.pdf [Accessed 1 October 2021] [Google Scholar]
- 17.Orkin C, Arasteh K, Górgolas Hernández-Mora M, Pokrovsky V, Overton ET, Girard PM, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382:1124–1135. [DOI] [PubMed] [Google Scholar]
- 18.Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382:1112–1123. [DOI] [PubMed] [Google Scholar]
- 19.Overton ET, Richmond G, Rizzardini G, Jaeger H, Orrell C, Nagimova F, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2 M), 48-week results: a randomised, multicentre, open-label, phase 3b, noninferiority study. Lancet 2021; 396:1994–2005. [DOI] [PubMed] [Google Scholar]
- 20.Margolis DA, Brinson CC, Smith GHR, de Vente J, Hagins DP, Eron JJ, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis 2015; 15:1145–1155. [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 22.Dixon JR, Jr. The International Conference on Harmonization Good Clinical Practice guideline. Qual Assur 1998; 6:65–74. [DOI] [PubMed] [Google Scholar]
- 23.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, Eron JJ, Yazdanpanah Y, Podzamczer D, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, noninferiority trial. Lancet 2017; 390:1499–1510. [DOI] [PubMed] [Google Scholar]
- 24.Kerrigan D, Sanchez Karver T, Muraleetharan O, Savage V, Mbwambo J, Donastorg Y, et al. A dream come true’: perspectives on long-acting injectable antiretroviral therapy among female sex workers living with HIV from the Dominican Republic and Tanzania. PLoS One 2020; 15:e0234666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weld ED, Rana MS, Dallas RH, Camacho-Gonzalez AF, Ryscavage P, Gaur AH, et al. Interest of youth living with HIV in long-acting antiretrovirals. J Acquir Immune Defic Syndr 2019; 80:190–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philbin MM, Parish CL, Kinnard EN, Reed SE, Kerrigan D, Alcaide ML, et al. Multisite study of women living with HIV's perceived barriers to, and interest in, long-acting injectable antiretroviral therapy. J Acquir Immune Defic Syndr 2020; 84:263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams J, Sayles HR, Meza JL, Sayre P, Sandkovsky U, Gendelman HE, et al. Long-acting parenteral nanoformulated antiretroviral therapy: interest and attitudes of HIV-infected patients. Nanomedicine (Lond) 2013; 8:1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dandachi D, Dang BN, Lucari B, Swindells S, Giordano TP. Acceptability and preferences for long-acting antiretroviral formulations among people with HIV infection. AIDS Care 2020; 33:801–809. [DOI] [PubMed] [Google Scholar]
- 29.Kerrigan D, Mantsios A, Gorgolas M, Montes ML, Pulido F, Brinson C, et al. Experiences with long acting injectable ART: a qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain. PLoS One 2018; 13:e0190487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray M, Antela A, Mills A, Huang J, Jäger H, Bernal E, et al. Patient-reported outcomes in ATLAS and FLAIR participants on long-acting regimens of cabotegravir and rilpivirine over 48 weeks. AIDS Behav 2020; 24:3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chounta V, Overton ET, Mills A, Swindells S, Benn PD, Vanveggel S, et al. Patient-reported outcomes through 1 year of an HIV-1 clinical trial evaluating long-acting cabotegravir and rilpivirine administered every 4 or 8 weeks (ATLAS-2 M). Patient 2021; doi.org/10.1007/s40271-021-00524-0 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.