Abstract
Objective
To determine the impact of dietary weight loss (WL) plus aerobic exercise (EX) and a “move more, more often” approach to activity promotion (SitLess; SL) on weight loss and maintenance.
Methods
Low-active, older adults (65–86 years) with obesity were randomized to WL+EX, WL+SL or WL+EX+SL. Participants received a social-cognitive group-mediated behavioral weight loss program for 6 months, followed by a 12-month maintenance period. EX participants received guided walking exercise with the goal of walking 150 minutes/week. SL attempted to achieve a step goal by moving frequently during the day. The primary outcome was month 18 body weight, with secondary outcomes including weight regain from 6–18 months, and objectively assessed physical activity and sedentary behavior at each timepoint.
Results
All groups demonstrated significant weight loss over 6 months (ps<.001) with no group differences. Groups that received SL improved total activity time (ps≤ .05), and those who received EX improved moderate-to-vigorous activity time (ps=.003). Over the 12-month follow-up period, those who received WL+EX demonstrated greater weight regain (5.2kg; 95%CI 3.5,6.9) relative to WL+SL (2.4kg; 95%CI 0.8,4.0).
Conclusion
Pairing dietary weight loss with a recommendation to accumulate physical activity contributed to similar weight loss and less weight regain compared with traditional aerobic exercise.
Keywords: Physical activity, Aging, Obesity, Weight Regain
Introduction
Obesity is highly prevalent among older adults (1) and is problematic in this age group as it accelerates decline in physical function and, thus, is a major risk factor for major mobility disability (2–4). Current obesity treatment guidelines for all ages recommend an intensive lifestyle intervention involving a reduced calorie diet, increased physical activity, and behavior change counseling to achieve weight loss (WL), followed by participation in a high-volume of at least moderate intensity physical activity (MVPA) to prevent weight regain (5). Whereas physical activity is often promoted via structured exercise, this strategy has been questioned as effective for sustaining WL (6). This may be especially true for older adults who may engage in compensation (i.e., increased sedentary behavior [SB]) and substitution (i.e., decreased time spent in non-exercise physical activity) when adopting structured exercise (7). This may result in a counter-productive reduction in total energy expenditure in the short term (8–11), and predispose individuals to a poorer overall profile of physical activity, marked by fewer minutes of daily activity and more SB, as they transition out of a formal activity program. Additionally, individuals with obesity and compromised physical function may find they are unable to sustain intense exercise (12) or may find it aversive (13).
Within the last decade, a body of research has emerged demonstrating that achieving recommended levels of physical activity through frequent, short bouts of activity produces similar or better health outcomes as participating in discrete bouts of conventional structured exercise (14–16). Previous intervention data collected on older adults suggests targeting frequent movement contributes to enhanced physical functioning,(17, 18) improved self-efficacy and quality of life,(19) and short-term weight loss as well as improved weight maintenance.(18, 20) Moreover, integrating enjoyable movement into daily life is likely to be more attainable for individuals with different functional abilities and preferences, and has the added benefit of indirectly breaking up sedentary behavior, and breaking prolonged sedentary time is widely associated with a host of favorable cardiometabolic health outcomes.(21, 22) In light of this evidence, the US physical activity guidelines were revised in 2018 to reflect the importance of a “move more, more often” approach to physical activity promotion.(16) A critical and timely question for clinical medicine is whether a recommendation to move more frequently throughout the day has benefit over a traditional aerobic exercise recommendation for producing WL and WL maintenance in older adults with obesity.
The purpose of this 18-month randomized clinical trial in older adults with obesity was to compare a behavioral dietary WL treatment paired with three different physical activity interventions on WL and weight regain following an intensive intervention period. Specifically, dietary WL was combined with (a) structured aerobic exercise (WL+EX), (b) an intervention focused on accumulating total activity and reducing SB through frequent daily movement (WL+SitLess; SL), or (c) WL+EX+SL. We expected that the addition of the SL intervention to a standard WL and EX program would result in a lower body weight at 18 months (primary outcome) and less weight regain following intervention (weight regain from 6 to 18 months; secondary outcome), and that WL+SL would be as effective as WL+EX.
Methods
The Institutional Review Board of the Wake Forest School of Medicine approved all study related procedures. All interested and potential participants signed an approved informed consent and HIPAA authorization form before any data collection. The Empowered with Movement to Prevent Obesity and Weight Regain (EMPOWER) study was an 18-month, 3-group, single-blind, randomized trial (Trial Registration: NCT02923674) as recently described in a study design publication.(23) Recruitment occurred in six waves between 2016 and January 2019, and final follow-up completed in September 2020. Participants recruited in the first four waves of this study completed procedures prior to onset of the COVID-19 pandemic in the United States, while the final two waves participated during the COVID-19 quarantine orders. The analyses presented herein pertain to participants who completed study procedures prior to the COVID-19 quarantine orders.
Participant Identification
Men and women from Forsyth County, NC and surrounding areas were recruited through local advertisements (see Online Supplement for more information on recruitment and representation). Our recruitment goal was 180 men and women aged 65–85 years who were classified as obese based on a BMI of 30–45 kg/m2.
Inclusion and Exclusion Criteria
In addition to the age and BMI criteria, eligibility criteria included: 1) insufficiently active (i.e., no participation in regular resistance training and/or > 20 mins/day of aerobic exercise in past 6 months); 2) non-smoking for >1 year; 3) <5% weight change in past 6 months; and 4) no insulin-dependent or uncontrolled diabetes (fasting glucose >140 mg/dl), osteoporosis (self-reported or t-score < −2.3 on hip or spine DXA scan), cognitive impairment (Montreal Cognitive Assessment <22), or clinical evidence of depression, anemia, heart disease, cancer, liver, renal, or chronic pulmonary disease, uncontrolled hypertension (>160/90 mmHg), major physical impairment, or contraindication for exercise or weight loss. These criteria were selected to minimize the likelihood for adverse events related to dietary weight loss and/or participation in moderate-to-vigorous physical activity. Participants were also asked about their access to a personal smartphone device and willingness to use it in the study. Those without a device or without consistent access to mobile internet were provided with a smartphone for the duration of the study (33/183; 18%).
A total of 1655 participants were screened by telephone to assess general eligibility criteria (see Consolidated Standards of Reporting Trials [CONSORT] diagram in Figure 1). Of those, 336 provided informed consent to complete baseline screening, which involved a medical history review, physical exam, cognitive and depression screening, fasting blood draw, and 12-lead resting electrocardiogram followed by a cardiopulmonary exercise test (CPET) to exhaustion.
Figure 1.
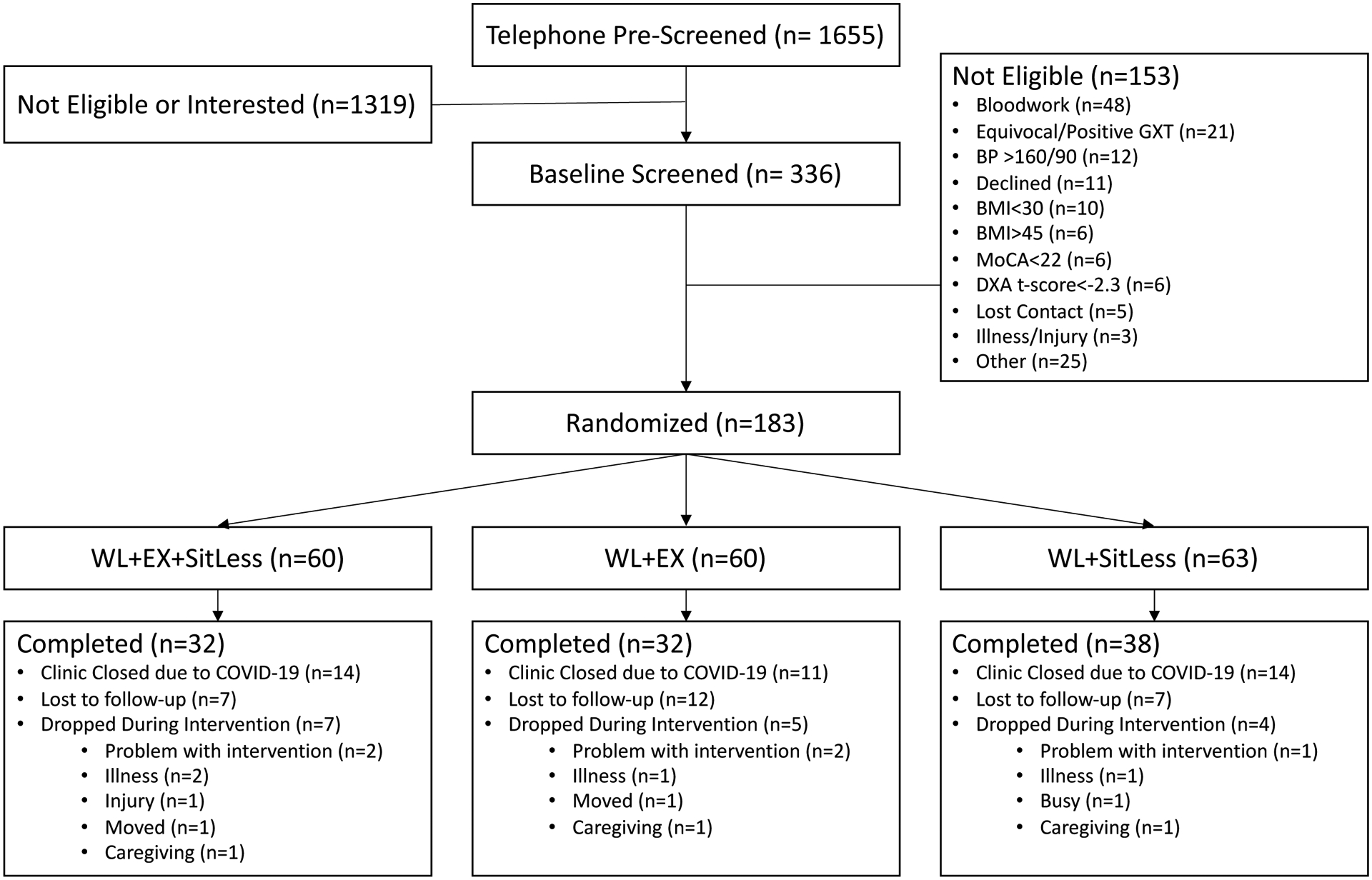
Participant Consort Diagram. BP = blood pressure, BMI =body mass index, MoCA=Montreal Cognitive Assessment, GXT = graded exercise test, DXA= dual-energy X-ray absorptiometry, WL=weight loss, EX = aerobic exercise.
Randomization
A total of 183 participants met all study eligibility criteria and were randomized 1:1:1 to one of the three treatment arms using a web-based randomization scheme developed by the study biostatistician (Figure 1). Randomization was stratified by gender with random block sizes. A total of 120 participants received the intervention and assessments per protocol prior to COVID-19 stay-at-home orders.
Interventions
The EMPOWER mHealth Toolset
All participants received a Fitbit Alta activity monitor at least two weeks prior to the start of the intervention, and the device was paired with a mobile health application that was tailored to each intervention arm (mHealth app; the EMPOWER Companion App)(23). The app facilitated contact between group members and research staff between intervention visits and was designed to facilitate self-monitoring of activity behaviors by providing group-specific visual feedback of Fitbit activity data, which is described further below.
Dietary Weight Loss (WL)
All participants underwent a diet intervention designed to elicit 7–10% WL from baseline body mass. Individual goals for caloric intake were prescribed to achieve an energy deficit of ~400 kcal/d from weight maintenance energy requirements (calculated as measured resting energy expenditure times an activity factor of 1.3). The macronutrient goal targeted an intake range of 25–30% from protein, 20–35% fat, and 45–55% carbohydrates.
In the first 6 months of the study, considered to be the intensive phase, participants attended weekly in-person group sessions by treatment arm delivered by the registered dietitian (RD) and a staff member with expertise in behavioral interventions. The group sessions were designed using principles from social cognitive theory (24) and the group dynamics literature, (25) with an emphasis on developing self-regulatory skills, social support, nutritional knowledge, and an awareness of daily dietary patterns via mindful eating exercises and food tracking. Body weight was measured and recorded at all sessions. In addition, participants were asked to track their daily food and beverage intake and these logs were reviewed weekly by the RD. Participants were allowed to reduce their frequency of logging at the discretion of the RD. For the final month of the intensive phase, focus was placed on transitioning toward self-management of WL, with an emphasis on continued self-monitoring and relapse prevention. During the transition phase of the study (months 7–9) group sessions were held twice monthly and participants were asked to continue logging their food/beverage intake and body weight. Group sessions were not held during the maintenance phase (months 10–18), but monthly contact was maintained with participants, either by brief phone call or email, to encourage study retention.
The SitLess Day-Long Movement Program
Participants in the WL+SL and WL+EX+SL treatment arms aimed to indirectly reduce the presence of sustained sitting bouts by engaging in frequent bouts of physical activity. These sessions occurred in conjunction with the diet sessions and followed the same contact schedule to achieve treatment goals (weekly during the first 6 months and bimonthly during months 7–9). During the maintenance phase (months 10–18), the monthly phone call or email contact emphasized adherence to the SL goals.
SitLess group content focused on optimizing patterns of movement such that a daily step goal was achieved by evenly distributing stepping throughout the day. This was monitored using the Companion mHealth app, which displayed progress toward step goals, and minute-level Fitbit data were displayed on a daily timeline bar in near real time (see Online Supplemental Figure S1). Daily stepping goals were increased by approximately 25% each week in collaboration with an interventionist until a maintenance limit of 10,000 steps was achieved. See “Goal Setting and the Companion App” in the online supplement for more detail. Intervention leaders also provided guidance and motivation to achieve movement throughout the day at home (e.g., stand and complete light movement while watching television, finding a space to engage in mindful walking), and in the community (e.g., identify opportunities for active transport). Of note, we have retained the SitLess label here to align with previously published work,(23) but ongoing iterations of this project have adopted the phrasing of “day-long physical activity” to better reflect the nature of the intervention.
Aerobic Exercise
Participants in the WL+EX and WL+EX+SL treatment arms aimed to perform structured aerobic exercise (treadmill walking) of moderate intensity for 4–5 days/week, progressing to a duration of 200 min/week. Participants were asked to attend center-based sessions for at least 3 days/week during the 6-month intensive phase and at least 1 day/week during the 3-month transition phase (months 7–9), exercising at home for the other 2–4 days/week. During the maintenance phase, the monthly phone call or email contact emphasized adherence to the moderate-intensity exercise goal of 5–6 days/week to maintain a volume of 200 min/week.
The supervised exercise sessions consisted of treadmill walking in which participants began with a 3–5 min warm-up at a slow pace before progressing to an intensity of 65–70% of their heart rate reserve (assessed during their baseline CPET). The exercise duration progressed to 40–50 min by the end of the 6th week and thereafter. Two heart rate readings were taken during each supervised session to monitor compliance to the prescribed intensity, and speed and grade were adjusted as necessary to ensure that participants exercised at their prescribed intensity. Each walking session ended with a 3–5 min cool-down followed by 5 min of large muscle flexibility stretches.
Regarding exercise conducted outside of the center, participants received guidance on how to safely complete ground or treadmill walking, elliptical, or stationary cycling depending on availability. They were also advised to sustain moderate-intensity activity equivalent to a rating of 13–15 using Borg’s Rating of Perceived Exertion (RPE). As a self-monitoring tool, participants were instructed to self-report their completion of their daily exercise bouts using the Companion mHealth app, wherein they checked “I Exercised Today”. They were also able to view their participation in sustained bouts of activity of at least 10 minutes within a timeline bar in the app (see Online Supplemental Figure S1). For individuals in the WL+EX+SL condition, the EMPOWER Companion App integrated feedback and self-monitoring tools pertaining to both moving throughout the day and achieving exercise goals, and this is described in detail in the passage “Goal Setting and the Companion App” within the Online Supplement.
Outcomes
Study assessments were collected by blinded research staff at baseline, after the intensive phase of intervention (6 months), and at the end of the maintenance phase (18 months).
Body Weight
The primary outcome of body weight was measured on the same scale within our clinical research center.
Physical Activity and Sedentary Behavior
Physical activity and sedentary behavior were assessed using the ActivPAL™ monitor (PAL Technologies, Glasgow, Scotland), which provides an accurate assessment of stepping(26) and sedentary behaviors.(27) Devices were initialized to record data at 20Hz and were worn continuously on the non-dominant thigh for seven days at each time point (i.e., baseline, 6 months, 18 months). Data were downloaded at the end of each seven-day wear period using the PAL studio software suite (version 8). Variables of interest include average daily time spent stepping (i.e., total PA), as well as time spent stepping for at least 10 minutes. We also report average daily minutes spent above 100 steps/minute (a proxy for MVPA),(28) and average daily minutes spent sitting. We computed minutes of light-intensity PA [LPA] as the difference between total PA and MVPA.
Additional Measures
Baseline demographic, medical history, and medication use data were recorded based on participant self-report. Resting metabolic rate (RMR) was used to determine each participant’s weight-maintaining energy needs for prescribing the 400 kcal/d energy deficit for WL (see Online Supplement for RMR procedures). Body composition was measured by dual-energy x-ray absorptiometry (DXA; at baseline, 6 and 18 months only). The Short Physical Performance Battery (29), a measure of lower-extremity physical function consisting of walking speed, balance, and repeated chair stands, was collected at baseline to characterize the functional status of participants. Finally, we noted several measures of adherence to key intervention tools, including attendance at group meetings, planned exercise contacts (EX groups only), and mHealth application accesses.
Statistical Analyses
The study was initially designed to provide >85% statistical power with an estimated 51 completers per condition to compare group differences of 2.7kg in body weight at 18 months considering two comparisons: (1) WL+EX+SL vs. WL+EX; and (2) WL+EX+SL vs. WL+SL; a conservative group difference based upon our previous pilot work.(20, 23) However, due to the COVID-19 pandemic, only the participants from the first four waves completed the intervention as originally designed, reducing our sample size to 112 completers of 120 randomized. This sample size provides a power of 64% to detect the pre-specified body weight difference, and a power of 80% to detect a larger group difference of 3.2 kg of body weight at 18 months.
For our primary outcome—body weight at 18 months—analysis of covariance (ANCOVA) was used to compare group differences adjusted for baseline weight, age, and gender.(30) If the overall test was statistically significant, we compared conditions via two-sided tests at α = .025 to maintain an overall type I error rate of 0.05. As secondary analyses, we used ANCOVA to compare group difference in 6-month body weight (i.e., after the intensive intervention phase). In addition, we were also interested in comparing weight regain from the end of the intensive intervention to the end of the maintenance period (6–18 months). For these analyses, we used ANCOVA to compare weight regain between groups from 6 to 18 months adjusted for age, gender, and weight loss from 0 to 6 months. These analyses were replicated for lean and fat mass collected via DXA and physical activity outcomes collected via the ActivPAL (i.e., average daily minutes of total PA, MVPA, light PA, and sedentary time). Unadjusted changes in each outcome were also tested using student t-tests. Statistical significance was established at p < .05.
Results
Retention, adherence, and baseline characteristics
The baseline characteristics of randomized participants are shown by treatment group in Table 1 and in the CONSORT diagram depicted in Figure 1. The average participant age was 70.0±4.7 years and participants were classified as obese (BMI=35.4±3.6 kg/m2), and primarily female (81.7%), white (78.3%) and highly educated, with hypertension and arthritis being the most prevalent self-reported comorbidities. These traits did not statistically differ between study groups.
Table 1.
Baseline demographic and health characteristics by intervention group.
| Overall (N=120) |
WL+EX N=40 |
WL+SL N=41 |
WL+EX+SL N=39 |
|
|---|---|---|---|---|
| Age; years (m±SD) | 70.0 ± 4.7 | 70.9 ± 4.4 | 69.6 ± 5.2 | 69.4 ± 4.4 |
| Female; n(%) | 98 (81.7%) | 34 (85.0%) | 32 (78.0%) | 32 (82.1%) |
| White; n(%) | 94 (78.3%) | 32 (80.0%) | 33 (80.5%) | 29 (74.4%) |
| Education (> High School); n(%) | 102 (85.0%) | 34 (85.0%) | 36 (87.8%) | 32 (82.1%) |
| CES-D; (m±SD) | 6.2 ± 5.8 | 7.6 ± 7.1 | 5.2 ± 5.2 | 5.7 ± 4.7 |
| Body Mass Index; kg/m2 (m±SD) | 35.4 ± 3.6 | 34.9 ± 3.3 | 35.8 ± 3.8 | 35.5 ± 3.7 |
| Waist to Hip Ratio; (m±SD) | 0.9 ± 0.2 | 0.9 ± 0.1 | 1.0 ± 0.3 | 0.9 ± 0.1 |
| VO2 Peak; ml/kg/min (m±SD) | 19.0 ± 3.9 | 19.2 ± 3.1 | 18.7 ± 4.5 | 19.1 ± 3.9 |
| Hypertension; n(%) | 81 (67.5%) | 31 (77.5%) | 25 (61.0%) | 25 (64.1%) |
| Diabetes; n(%) | 15 (12.5%) | 4 (10.0%) | 8 (19.5%) | 3 (7.7%) |
| Sleep Apnea; n(%) | 41 (34.2%) | 12 (30.0%) | 9 (22.0%) | 20 (51.3%) |
| Osteoarthritis; n(%) | 82 (68.3%) | 27 (67.5%) | 30 (73.2%) | 25 (64.1%) |
| Osteopenia; n(%) | 30 (25.0%) | 12 (30.0%) | 7 (17.1%) | 11 (28.2%) |
Notes: M=mean; SD=standard deviation; WL=Weight Loss; EX=aerobic exercise; SL=SitLess; MoCA=Montreal Cognitive Assessment; CES-D=Center for Epidemiological Studies Depression Scale;
Group meeting attendance was 80% in WL+SL, 84% in WL+EX, and 78% in WL+EX+SL. Regarding structured exercise appointments, those in WL+EX attended 79% of exercise sessions on average while WL+EX+SL attended 65%. Average weekly application usage during the intensive phase (weeks 1–24) and the full study period (weeks 1–72) are displayed in Table 2. Those who received the SL (i.e., WL+EX+SL, WL+SL) intervention tended to view their app more frequently, which was expected as these individuals were coached to view their activity patterns several times daily.
Table 2:
Average (SD) weekly application usage such that weekly application accesses were averaged for each participant during the intensive phase (weeks 1–24) and full study period (weeks 1–72).
| Group | Intensive Phase | Full Study |
|---|---|---|
| WL + SL (n=40) | 14.05 (14.86) | 10.01 (11.83) |
| WL + EX (n=40) | 10.74 (7.20) | 7.10 (5.75) |
| WL + SL + EX (n=39) | 16.30 (28.04) | 10.66 (19.49) |
Notes: WL = weight loss; EX = aerobic exercise; SL = SitLess; M = mean; SD = standard deviation
Primary Outcome (Weight Loss at Month 18)
Unadjusted body weights are displayed in Table 3, whereas body weights at 6 and 18 months, adjusted for baseline values, gender, and age are displayed in Table 4 and Figure 2. There was no significant group difference in adjusted body weight at 18 months (p=.510), though all groups lost significant body weight between 0 and 6 months (ps < .001) and between 0 and 18 months (ps ≤ .002). Average group weight loss at 18 months ranged between −4.3 kg (WL+EX) to −6.4 kg (WL+SL) from baseline.
Table 3.
Unadjusted body weight, lean mass, and fat mass (kg) at baseline, following the 6-month intensive phase, and following the maintenance phase (18 months)
| Condition | Baseline (M±SD) | Month 6 (M±SD) | Month 18 (M±SD) |
|---|---|---|---|
| Body Weight | |||
| WL + EX (n=31) | 91.6±14.3 | 82.8±11.4 | 87.2±13.1 |
| WL + SL (n=33) | 95.1±14.3 | 87.0±13.3 | 88.7±14.3 |
| WL + EX + SL (n=26) | 94.7±10.2 | 86.9±12.4 | 89.8±14.3 |
| All (n=90) | 93.8 ±13.2 | 85.6±12.5 | 88.5±13.7 |
| Lean Mass | |||
| WL + EX (n=31) | 47.4±10.7 | 45.0±9.6 | 45.9±10.3 |
| WL + SL (n=33) | 48.8±9.8 | 46.3±9.5 | 46.9±9.7 |
| WL + EX + SL (n=26) | 49.3±7.5 | 47.2±7.7 | 47.4±7.7 |
| All (n=90) | 48.5±9.5 | 46.1±9.0 | 46.7±9.3 |
| Fat Mass | |||
| WL + EX (n=31) | 43.0±5.2 | 37.3±5.1 | 41.0±5.4 |
| WL + SL (n=33) | 45.3±7.3 | 39.7±7.0 | 41.0±7.3 |
| WL + EX + SL (n=26) | 44.6±8.0 | 39.5±9.2 | 41.3±0.8 |
| All (n=90) | 44.3±6.9 | 38.8±7.2 | 41.1±7.7 |
Notes: WL = weight loss; EX = aerobic exercise; SL = SitLess; M = mean; SD = standard deviation
Table 4.
Adjusted body weight, lean mass, and fat mass (kg) following the 6-month intensive phase, the 18-month maintenance phase, and regain in adjusted weight between 6 and 18 months.
| Condition | Month 6 M (95% CI) | Month 18 M (95% CI) | Weight Regain M (95% CI) |
|---|---|---|---|
| Body Weight | |||
| WL + EX (n=31) | 84.3 (82.1,86.5) | 89.5 (86.7,92.3) | 5.2 (3.5,6.9) |
| WL + SL (n=33) | 85.5 (83.3, 87.6) | 87.7 (84.9,90.4) | 2.4 (0.8,4.0) |
| WL + EX + SL (n=26) | 85.6 (82.1, 88.0) | 89.2 (86.1,92.3) | 3.8 (2.0,5.6) |
| p | 0.606 | 0.510 | 0.035 |
| Lean Mass | |||
| WL + EX (n=31) | 46.3 (45.5,47.0) | 47.2 (46.2,48.1) | 0.7 (0.2,1.3) |
| WL + SL (n=33) | 46.0 (45.3,46.8) | 46.9 (46.0,47.8) | 0.6 (0.0,1.1) |
| WL + EX + SL (n=26) | 46.7 (45.8,47.5) | 47.4 (46.2,48.6) | 0.6 (−0.0,1.2) |
| p | 0.281 | 0.593 | 0.892 |
| Fat Mass | |||
| WL + EX (n=31) | 37.6 (36.0,39.2) | 42.1 (40.0,44.3) | 4.8 (3.5,6.1) |
| WL + SL (n=33) | 38.1 (36.6,39.7) | 40.4 (38.4,42.4) | 2.2 (1.1,3.4) |
| WL + EX + SL (n=26) | 38.3 (36.7,40.0) | 40.9 (38.5,43.2) | 3.4 (2.0,4.8) |
| p | 0.765 | 0.429 | 0.006 |
Notes: Follow-up body weights adjusted for age, gender, and baseline body weight; WL = weight loss; EX = aerobic exercise; SL = SitLess; M = mean; CI = confidence interval.
Figure 2.
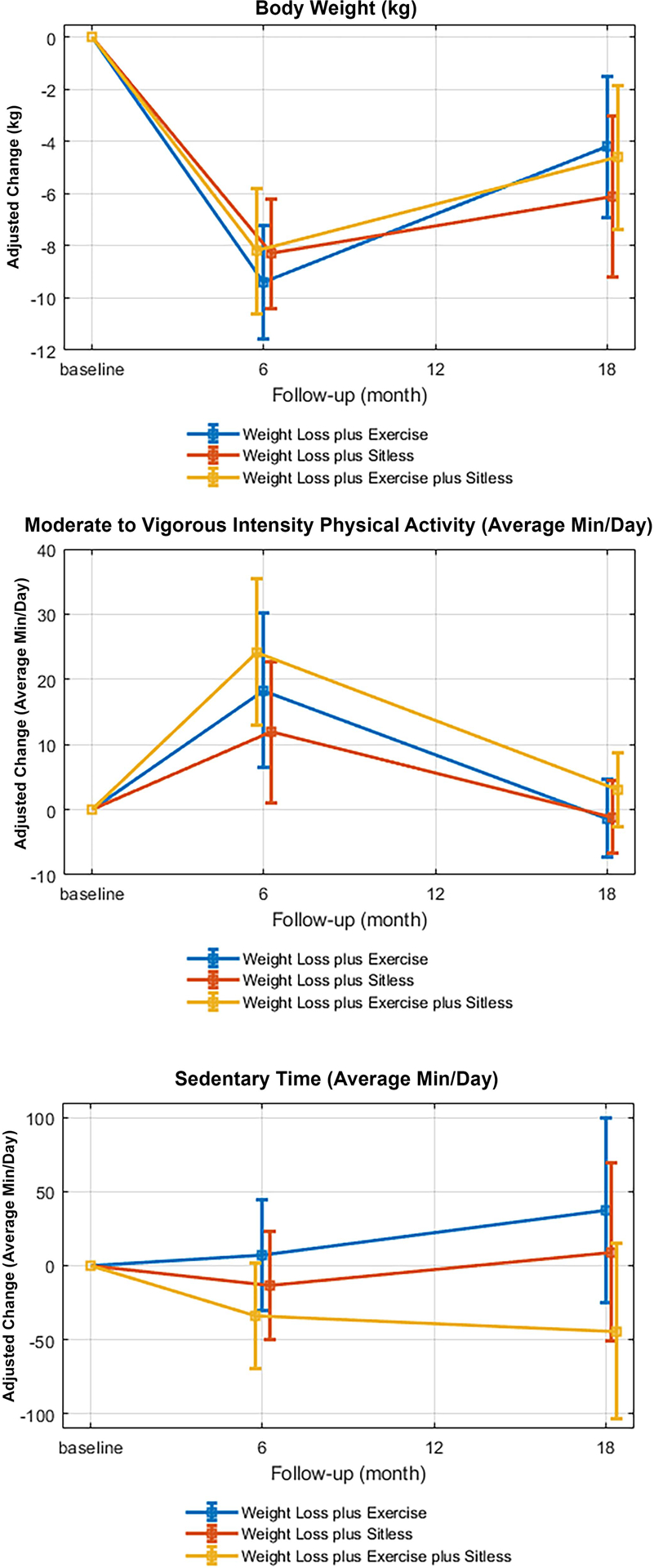
Adjusted change in body weight (top panel), average minutes of moderate-to-vigorous activity (MVPA; middle panel), and sitting time (bottom panel) within weight loss + exercise (n=13), weight loss + SitLess (n = 14), and weight loss + exercise + SitLess (n = 15).
Secondary Comparisons
Adjusted weight regain data are displayed in Table 4. There was a significant group difference in adjusted weight regain between 6 and 18 months (p=.035), which was driven by a difference between WL + EX and WL + SL whereby those in WL + EX regained 2.8 kg more (95% CI −4.9, −0.7) relative to WL + SL. WL+EX+SL did not significantly differ from either group. Unadjusted fat and lean mass are displayed in Table 3, and lean and fat mass at 6 and 18 months, adjusted for baseline values, gender, and age are displayed in Table 4. As with weight regain, there was a significant group difference in regain of fat mass between 6 and 18 months (p = .006), which was marked by the greatest regain the WL+EX condition and the least regain in the WL+SL condition who demonstrated the lowest adjusted fat mass at month 18.
Unadjusted average daily minutes of PA, MVPA, Light PA, and sedentary time are displayed in Table 5. It is notable that only 42 participants had complete ActivPAL data at all timepoints. The primary cause of this was faulty rechargeable batteries caused by overcharging the devices (i.e., leaving the devices on the charger prior to administration). Minutes of physical activity and sedentary behavior at months 6 and 18, adjusted for baseline values, gender, and age are displayed in Table 6. Within-group changes in all activity variables are displayed in Supplemental Table S1, and adjusted percent change in MVPA and sedentary time are displayed in Figure 2. There were no significant differences between groups in any activity variable at 6 or 18 months. Within-group analyses (Supplemental Table S1) revealed a significant improvement in overall activity time in the WL+EX+SL condition over the 6-month intensive intervention period (p < .001). A similar, but non-significant (p = .052) effect was observed in the WL+SL condition. Those who received EX demonstrated significant improvements in MVPA and time spent in bouts of PA of 10+ minutes over the 6-month intensive phase (ps ≤ .001).
Table 5.
Unadjusted ActivPAL physical activity data (average daily minutes) at baseline, following the 6-month intensive- and 18-month maintenance-phases
| Condition | Baseline (M±SD) | Month 6 (M±SD) | Month 18 (M±SD) |
|---|---|---|---|
| Minutes of PA | |||
| WL + EX (n=13) | 92.7 ± 30.6 | 100.3 ± 36.1 | 87.7 ± 37.5 |
| WL + SL (n=14) | 78.6 ± 20.4 | 96.9 ± 35.8 | 84.9 ± 23.4 |
| WL + EX + SL (n=15) | 91.8 ± 24.5 | 113.5 ± 27.0 | 100.6 ± 33.9 |
| All (n=42) | 87.7 ± 25.6 | 103.9 ± 33.0 | 91.4 ± 32.1 |
| Minutes of Bouted PA | |||
| WL + EX (n=13) | 0.7 ± 1.5 | 14.0 ± 9.4 | 2.5 ± 7.2 |
| WL + SL (n=14) | 0.4 ± 1.2 | 6.5 ± 17.2 | 1.7 ± 3.7 |
| WL + EX + SL (n=15) | 1.5 ± 2.9 | 20.1 ± 19.9 | 3.0 ± 10.3 |
| All (n=42) | 0.9 ± 2.0 | 13.7 ± 17.0 | 2.4 ± 7.5 |
| Minutes of MVPA | |||
| WL + EX (n=13) | 9.2 ± 7.5 | 21.9 ± 11.3 | 7.0 ± 7.4 |
| WL + SL (n=14) | 6.7 ± 5.2 | 15.9 ± 26.6 | 6.2 ± 4.4 |
| WL + EX + SL (n=15) | 9.9 ± 8.9 | 29.1 ± 20.9 | 11.8 ± 15.0 |
| All (n=42) | 8.6 ± 7.3 | 22.5 ± 21.0 | 8.4 ± 10.3 |
| Minutes of Light PA | |||
| WL + EX (n=13) | 83.5 ± 31.0 | 78.4 ± 34.5 | 80.7 ± 34.8 |
| WL + SL (n=14) | 71.9 ± 19.2 | 81.0 ± 21.4 | 78.7 ± 22.1 |
| WL + EX + SL (n=15) | 81.9 ± 22.0 | 84.4 ± 26.7 | 88.8 ± 33.0 |
| All (n=42) | 79.1 ± 24.3 | 81.4 ± 27.3 | 82.9 ± 30.0 |
| Sedentary Minutes | |||
| WL + EX (n=13) | 613.6 ± 89.3 | 629.6 ± 75.7 | 650.2 ± 116.7 |
| WL + SL (n=14) | 679.6 ± 146.8 | 653.2 ± 122.8 | 664.1 ± 138.2 |
| WL + EX + SL (n=15) | 566.0 ± 77.4 | 552.9 ± 90.7 | 534.9 ± 112.0 |
| All (n=42) | 618.6 ± 116.2 | 610.1 ± 105.9 | 613.7 ± 133.7 |
Notes: WL = weight loss; EX = aerobic exercise; SL = SitLess; M = mean; SD = standard deviation; PA = physical activity; Bouted PA = any physical activity occurring in bouts of at least 10 minutes
Table 6.
Adjusted ActivPAL physical activity data (average daily minutes) following the 6-month intensive phase and the 18-month maintenance phase, and change over maintenance period
| Condition | Month 6 M (95% CI) |
Month 18 M (95% CI) |
Change 6M to 18M M (95% CI) |
|---|---|---|---|
| Minutes of PA | |||
| WL + EX (n=13) | 103.5 (87.9,119.1) | 85.8 (70.1,101.6) | −18.3 (−33.7,−2.8) |
| WL + SL (n=14) | 108.4 (94.1,122.7) | 94.0 (79.6,108.5) | −9.8 (−23.8,4.2) |
| WL + EX + SL (n=15) | 117.1 (102.3,131.9) | 99.5 (84.6,114.5) | −7.8 (−23.1,7.5) |
| p | 0.34 | 0.35 | 0.52 |
| Minutes of Bouted PA | |||
| WL + EX (n=13) | 17.0 (7.2,26.8) | 2.9 (−2.2,8.0) | −10.0(−15.3,−4.6) |
| WL + SL (n=14) | 8.6 (−0.2,17.5) | 1.7 (−2.9,6.3) | −11.2(−16.1,−6.2) |
| WL + EX + SL (n=15) | 20.1 (10.8,29.3) | 3.6 (−1.2,8.4) | −10.3(−15.5,−5.1) |
| p | 0.15 | 0.83 | 0.94 |
| Minutes of MVPA | |||
| WL + EX (n=13) | 27.0 (14.7, 39.3) | 7.3 (1.0,13.5) | −14.9 (−21.5,−8.3) |
| WL + SL (n=14) | 20.5 (9.3,31.7) | 7.5 (1.8,13.3) | −13.7 (−19.6,−7.8) |
| WL + EX + SL (n=15) | 32.8 (21.,44.5) | 11.6 (5.7,175) | −11.2 (−17.7,−4.7) |
| p | 0.26 | 0.43 | 0.64 |
| Minutes of Light PA | |||
| WL + EX (n=13) | 77.3 (64.0, 90.6) | 78.3 (63.4, 93.1) | −0.7 (−13.3,11.9) |
| WL + SL (n=14) | 88.0 (75.9, 100.1) | 85.7 (72.2, 99.2) | 0.4 (−11.1,11.9) |
| WL + EX + SL (n=15) | 84.6 (72.0, 97.2) | 87.9 (73.8,101.9) | 4.4 (−7.4,16.3) |
| p | 0.43 | 0.54 | 0.77 |
| Sedentary Minutes | |||
| WL + EX (n=13) | 625.6 (586.6,664.5) | 656.0 (591.8,720.2) | 29.5 (−24.5,83.5) |
| WL + SL (n=14) | 605.0 (56.1,64.9) | 627.8 (565.4, 690.2) | 16.3 (−33.1, 65.8) |
| WL + EX + SL (n=15) | 584.8 (547.7,622.0) | 574.5 (513.3,635.7) | −9.0 (−60.6,42.6) |
| p | 0.24 | 0.12 | 0.49 |
Notes: Minutes of each behavior adjusted for age, gender, and baseline body weight; WL = weight loss; EX = aerobic exercise; SL = SitLess; M = mean; CI = confidence interval; PA = physical activity; Bouted PA = any physical activity occurring in bouts of at least 10 minutes
Discussion
We hypothesized that the WL+EX+SL condition would demonstrate superior long-term weight loss and less weight regain relative to WL+EX and WL+SL. Counter to these hypotheses, we found that the addition of structured aerobic exercise to weight loss (i.e., WL+EX, WL+EX+SL) did not produce superior weight loss relative to WL+SL over either 6 or 18 months. Indeed, all conditions demonstrated significant and similar weight loss over the intensive 6-month intervention period. The only significant difference in weight regain was between the WL+SL and WL+EX conditions whereby the WL+SL condition regained the least amount of body weight. We observed similar findings in DXA-assessed fat mass. We also observed several interesting patterns in the ActivPAL data, which should be interpreted with caution given the small sample. We found that assignment to structured exercise increased MVPA time, while assignment to a day-long movement intervention resulted in greater total activity time. Also of interest, WL+EX demonstrated no improvement in total activity time, which may have adverse implications for long-term behavior and associated health outcomes. We observed an expected regression to baseline MVPA levels on cessation of a formal intervention across all conditions. However, only WL+EX demonstrated a non-significant decrease in light-intensity activity and increase in sedentary time by month 18 (see Figure 2 and Supplemental Table S1). Future work should investigate the impact of such an activity profile on longer-term weight changes in a larger sample.
Achieving a sufficient volume of MVPA nested within frequent bouts of LPA benefits a host of health-related outcomes including body weight,(31, 32) and current PA guidelines recommend a large volume of MVPA for preventing weight gain.(5) Naturally, realizing these benefits requires lasting participation in activity behavior.(33) A unique strength of our study is the inclusion of a long-term maintenance period, allowing for investigation of weight regain within three groups differing in the physical activity recommendation they received. We add to the growing body of evidence demonstrating that a recommendation to engage in supervised exercise often fails to support long-term weight loss maintenance. This may be due to compensatory behaviors such as increased sitting and decreased non-exercise activities.(7, 9, 34–38) Achieving daily activity goals across many brief bouts is one potential strategy for avoiding prolonged sitting and supporting participation in non-exercise activities. Moreover, a growing body of research suggests that avoiding prolonged bouts of inactivity offers unique health benefits such as enhanced glucose regulation and appetite regulation while being attainable for a broader range of older adults.(14, 16, 21, 39–41) We would like to emphasize the importance of not conflating non-exercise activity with LPA. While some individuals find structured exercise to be enjoyable, others may find more pleasure in a variety of alternative MVPAs of varied bout lengths (e.g., active transport, brief episodes of movement/working around the home). An optimal physical activity profile is one that embeds intrinsically motivating MVPA into frequent bouts of LPA while avoiding prolonged bouts of inactivity.(16)
The EMPOWER study had notable strengths in addition to those described above. It employed a novel physical activity protocol focused on the accumulation of activity across the day. This study provides preliminary evidence that this approach is helpful for guarding against increased sitting in response to structured exercise, though the small sample with complete ActivPAL data suggest this should be interpreted with caution. We have since conducted several iterative studies designed to refine this novel coaching model.(18, 42, 43) An important limitation was that the COVID-19 pandemic prevented collection of objective follow-up data in two planned waves, reducing our sample size. Our sample was comprised largely of female and white participants, potentially limiting generalizability. Additionally, EMPOWER did not include a WL-only control, and as such we cannot directly ascertain whether SL influenced weight loss and maintenance above and beyond the effect of the WL intervention on dietary intake. Likewise, it is important to recognize that EMPOWER employed a center-based, supervised exercise protocol. As such, we are unable to determine the impact of an unsupervised exercise intervention on weight loss/regain. Evidence suggests both approaches support weight loss to a similar degree (44) and that unsupervised exercise may produce better long-term adherence.(45) Future work should include an unsupervised exercise condition. Finally, there was a large proportion of participants without complete accelerometer data due to battery issues. This issue has been recognized by PAL Technologies, who now include instructions on preventing overcharging with new devices and have added battery health screening tools to their software.
Conclusion
Enrolling older adults in a WL+SL, WL+EX, or WL+EX+SL intervention contributes to significant weight loss. Counter to our expectations the WL+EX+SL condition did not lose more weight or contribute to better weight maintenance relative to WL+SL or WL+EX. Instead, only the WL+EX condition demonstrated significantly more regain relative to WL+SL. These results provide further support for recommending older adults “move more, more often” for minimizing weight regain after weight loss.
Supplementary Material
Figure S1. Daily Timeline Bar. Blue represents inactivity and green represents activity. Those in the exercise condition have bouts of at least 10 minutes of movement highlighted in bright green.
Study Importance.
What is already known?
Regular physical activity is important for weight loss maintenance.
Structured exercise often contributes to little change in overall daily energy expenditure due to increased sitting and/or decreased activities of daily living, especially in older adults.
What does this study add?
Combining behavioral dietary weight loss strategies with an intervention to increase movement throughout the day contributed to similar short term (6-month) weight loss when compared with behavioral dietary weight loss and structured aerobic exercise.
Those assigned to the daily movement intervention regained less weight after a year of follow-up compared to those who engaged in structured exercise.
How might these results change the focus of clinical practice?
Clinicians interested in supporting lasting weight loss should heed the advice of the US physical activity guidelines by recommending a “move more, more often” approach to physical activity.
Additional work is needed to integrate these principles into clinical weight management practices.
Acknowledgements
We are grateful to our participants, our Registered Dietitian Beverly Nesbit, our lead behavioral interventionist Sherri Ford, our lead exercise interventionist Terrell Wagner, and our coordinating and assessment team including Michelle Gordon, Justin Johnson, and Jessica Sheedy. Data collected for this study, including individual deidentified participant data and a data dictionary defining each field in the set, will be made available to others with an academic affiliation upon reasonable request from the corresponding author. The protocol for this trial has been previously published (23).
FUNDING:
This trial was funded by the National Institutes of Health and National Institute on Aging R01 AG051624. Dr. Fanning is also supported by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30-AG21332).
Footnotes
TRIAL REGISTRATION: ClinicalTrials.org Identifier: NCT02923674
DISCLOSURE: The authors declare no conflict of interest.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA 2014;311:806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rejeski WJ, Marsh AP, Chmelo E, Rejeski JJ. Obesity, intentional weight loss and physical disability in older adults. Obes Rev 2010;11:671–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Houston DK, Ding J, Nicklas BJ, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults: The health, aging and body composition study. Am J Epidemiol 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houston DK, Nicklas BJ, Zizza CA. Weighty Concerns: The Growing Prevalence of Obesity among Older Adults. J Am Diet Assoc 2009. [DOI] [PubMed] [Google Scholar]
- 5.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. 2018.
- 6.Foright RM, Presby DM, Sherk VD, et al. Is regular exercise an effective strategy for weight loss maintenance? Physiol Behav 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson D, Peacock OJ, Betts JA. Substitution and compensation erode the energy deficit from exercise interventions. Med Sci Sports Exerc 2014. [DOI] [PubMed] [Google Scholar]
- 8.Bonomi AG, Soenen S, Goris AHC, Westerterp KR. Weight-Loss Induced Changes in Physical Activity and Activity Energy Expenditure in Overweight and Obese Subjects before and after Energy Restriction. PLoS One 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melanson EL, Keadle SK, Donnelly JE, Braun B, King NA. Resistance to exercise-induced weight loss: Compensatory behavioral adaptations. Med Sci Sports Exerc 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin CK, Das SK, Lindblad L, et al. Effect of calorie restriction on the free-living physical activity levels of nonobese humans: Results of three randomized trials. J Appl Physiol 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redman LM, Heilbronn LK, Martin CK, et al. Metabolic and behavioral compensations in response to caloric restriction: Implications for the maintenance of weight loss. PLoS One 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rejeski WJ, Walkup MP, Fielding RA, et al. Evaluating Accelerometry Thresholds for Detecting Changes in Levels of Moderate Physical Activity and Resulting Major Mobility Disability. Journals Gerontol Ser A 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King AC, Rejeski WJ, Buchner DM. Physical activity interventions targeting older adults. Am J Prev Med 1998;15:316–333. [DOI] [PubMed] [Google Scholar]
- 14.Murphy MH, Lahart I, Carlin A, Murtagh E. The Effects of Continuous Compared to Accumulated Exercise on Health: A Meta-Analytic Review. Sport Med 2019;49:1585–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Physical Activity Guidelines Advisory Committee. 2018 physical activity guidelines advisory committee report. Washington, DC US Dep Heal Hum Serv; 2018. [Google Scholar]
- 16.Piercy KL, Troiano RP, Ballard RM, et al. The physical activity guidelines for Americans. JAMA 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.BG B, B JS, B T, et al. Reducing Sedentary Behavior Versus Increasing Moderate-to-Vigorous Intensity Physical Activity in Older Adults. J Aging Health 2017;29:247–267. [DOI] [PubMed] [Google Scholar]
- 18.Fanning J, Brooks A, Ip E, et al. A Mobile Health Behavior Intervention to Reduce Pain and improve Health in Older Adults with Obesity and Chronic Pain: The MORPH pilot trial. Front Digit Heal 2020;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fanning J, Brooks AK, Hsieh KL, et al. The Effects of a Pain Management-Focused Mobile Health Behavior Intervention on Older Adults’ Self-efficacy, Satisfaction with Functioning, and Quality of Life: a Randomized Pilot Trial. Int J Behav Med 2021:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicklas BJ, Gaukstern JE, Beavers KM, Newman JC, Leng X, Rejeski WJ. Self-monitoring of spontaneous physical activity and sedentary behavior to prevent weight regain in older adults. Obesity 2014;22:1406–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2008;31:369–71. [DOI] [PubMed] [Google Scholar]
- 22.Chastin SFM, Egerton T, Leask C, Stamatakis E. Meta-analysis of the relationship between breaks in sedentary behavior and cardiometabolic health. Obesity 2015;23:1800–1810. [DOI] [PubMed] [Google Scholar]
- 23.Fanning J, Opina MT, Leng I, Lyles MF, Nicklas BJ, Rejeski WJ. Empowered with Movement to Prevent Obesity & Weight Regain (EMPOWER): Design and methods. Contemp Clin Trials 2018;72:35–42. [DOI] [PubMed] [Google Scholar]
- 24.Bandura A Self-efficacy: The exercise of control. New York, NY: W. H. Freeman and Company; 1997. [Google Scholar]
- 25.Brawley LR, Rejeski WJ, Lutes L. A group-mediated cognitive behavioural intervention for increasing adherence to physical activity in older adults. J Appl Biobehav Res 2000;5:47–65. [Google Scholar]
- 26.Grant PM, Dall PM, Mitchell SL, Granat MH. Activity-Monitor Accuracy in Measuring Step Number and Cadence in Community-Dwelling Older Adults. J Aging Phys Act 2008;16:201–214. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y, Barry VW, Kang M. Validation of the ActiGraph GT3X and activPAL Accelerometers for the Assessment of Sedentary Behavior. 10.1080/1091367X20151054390 2015;19:125–137. [DOI] [Google Scholar]
- 28.Tudor-Locke C, Han H, Aguiar EJ, et al. How fast is fast enough? Walking cadence (steps/min) as a practical estimate of intensity in adults: A narrative review. Br J Sports Med 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–M94. [DOI] [PubMed] [Google Scholar]
- 30.Kahan BC, Jairath V, Doré CJ, Morris TP. The risks and rewards of covariate adjustment in randomized trials: an assessment of 12 outcomes from 8 studies. Trials 2014 151 2014;15:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bann D, Hire D, Manini T, et al. Light Intensity Physical Activity and Sedentary Behavior in Relation to Body Mass Index and Grip Strength in Older Adults: Cross-Sectional Findings from the Lifestyle Interventions and Independence for Elders (LIFE) Study. PLoS One 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O DM, L K, P Z, et al. Objectively Measured Physical Activity and Sedentary Behavior in Successful Weight Loss Maintainers. Obesity (Silver Spring) 2018;26:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc 2009. [DOI] [PubMed] [Google Scholar]
- 34.Di Blasio A, Ripari P, Bucci I, et al. Walking training in postmenopause: Effects on both spontaneous physical activity and training-induced body adaptations. Menopause 2012. [DOI] [PubMed] [Google Scholar]
- 35.Goran MI, Poehlman ET. Endurance training does not enhance total energy expenditure in healthy elderly persons. Am J Physiol - Endocrinol Metab 1992. [DOI] [PubMed] [Google Scholar]
- 36.Morio B, Montaurier C, Pickering G, et al. Effects of 14 weeks of progressive endurance training on energy expenditure in elderly people. Br J Nutr 1998. [DOI] [PubMed] [Google Scholar]
- 37.Meijer EP, Westerterp KR, Verstappen FTJ. Effect of exercise training on total daily physical activity in elderly humans. Eur J Appl Physiol Occup Physiol 1999. [DOI] [PubMed] [Google Scholar]
- 38.Melanson EL. The effect of exercise on non-exercise physical activity and sedentary behavior in adults. Obes Rev 2017;18:40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fanning J, Rejeski WJ, Chen S-H, et al. A Case for Promoting Movement Medicine: Preventing Disability in the LIFE Randomized Controlled Trial. Journals Gerontol Ser A 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dunstan DW, Howard B, Healy GN, Owen N. Too much sitting: a health hazard. Diabetes Res Clin Pract 2012;97:368–76. [DOI] [PubMed] [Google Scholar]
- 41.Hopkins M, Blundell JE. Energy balance, body composition, sedentariness and appetite regulation: pathways to obesity. Clin Sci 2016;130:1615–1628. [DOI] [PubMed] [Google Scholar]
- 42.Fanning J, Brooks AK, Hsieh KL, et al. Building on Lessons Learned in a Mobile Intervention to Reduce Pain and Improve Health (MORPH): Protocol for the MORPH-II Trial. JMIR Res Protoc 2021;10(7)e29013 https//www.researchprotocols.org/2021/7/e29013 2021;10:e29013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fanning J, Brooks AK, Ip E, Nicklas BJ, Rejeski WJ. A Mobile Health Intervention to Reduce Pain and Improve Health (MORPH) in Older Adults With Obesity: Protocol for the MORPH Trial. JMIR Res Protoc 2018;7:e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jakicic JM, Rogers RJ, Davis KK, Collins KA. Role of Physical Activity and Exercise in Treating Patients with Overweight and Obesity. Clin Chem 2018;64:99–107. [DOI] [PubMed] [Google Scholar]
- 45.Ashworth NL, Chad KE, Harrison EL, Reeder BA, Marshall SC, Group CM. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev 2005;2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Daily Timeline Bar. Blue represents inactivity and green represents activity. Those in the exercise condition have bouts of at least 10 minutes of movement highlighted in bright green.


