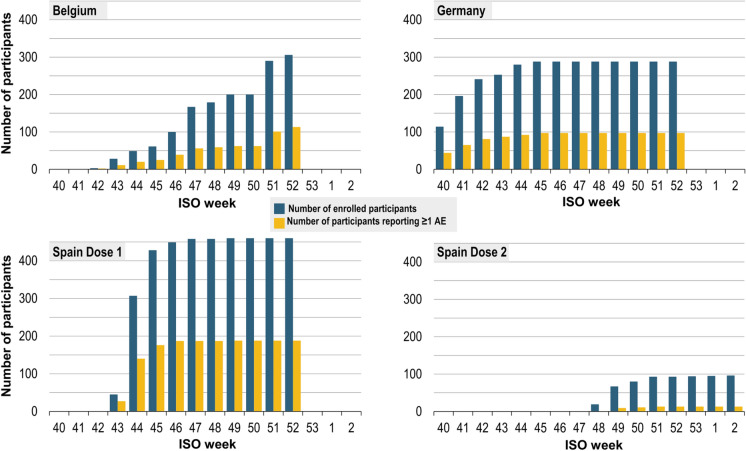
Fig. 2.
Number of enrolled participants (cumulative, by ISO week) with proportion reporting at least one AE after dose 1 or dose 2 (safety set). AE, adverse event; ISO, International Organization for Standardization. Cumulative number of enrolled participants by week and by country, with the proportion reporting at least one AE. Participants in Germany and Belgium were adults and therefore received only one dose of GSK’s inactivated quadrivalent seasonal influenza vaccine, while some participants in Spain were children eligible for two doses. Participants were enrolled from ISO week 40, 2020 (28 September–4 October 2020) to ISO week 2, 2021 (11–17 January 2021)