To the Editor: A 37-year-old Burmese woman presented with tender nodules of 6-week duration on the distal aspect of her legs, which appeared a day after receiving the second dose of tozinameran vaccination. She previously had erythema nodosum (EN) 10 years ago, which was related to pulmonary tuberculosis (PTB). She had completed treatment for PTB at that time, and there was a complete resolution of the prior episode of EN till the time of presentation. A computed tomography scan of her thorax obtained several months prior showed no evidence of active PTB, and a thorough review of systems was unyielding. Her medical history was significant for well-controlled Hashimoto thyroiditis.
On examination, there were erythematous, tender, nonulcerative deep nodules around both knees, on the right shin, and above the lateral aspect of the left malleolus (Fig 1). A lesional punch biopsy revealed septal panniculitis with granulomatous aggregates of foreign body–type multinucleated giant cells, histiocytes, and lymphocytes (Fig 2, A and B). There was no vasculitis or areas of necrosis, and infective stains, namely the Ziehl-Neelsen stain, did not reveal any microorganisms.
Fig 1.
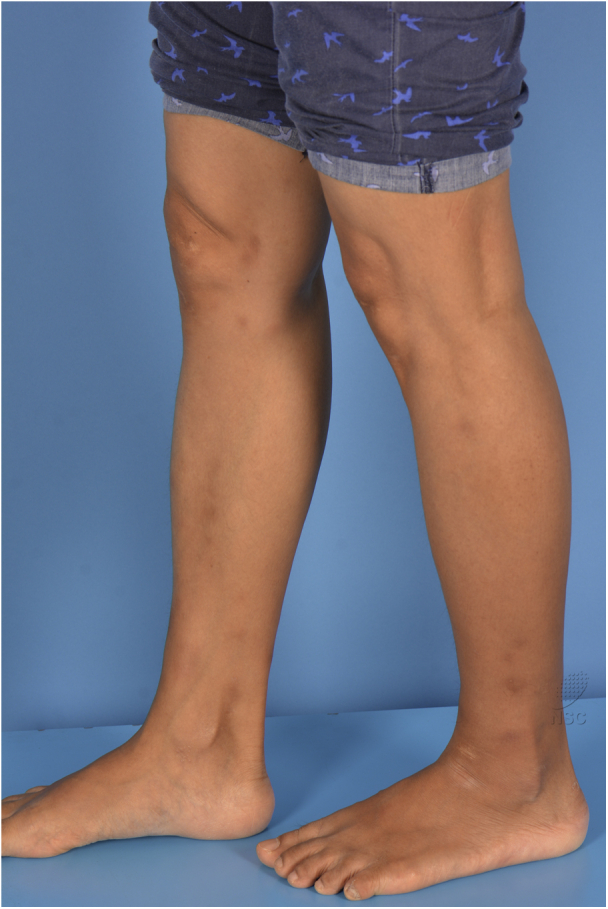
Erythema nodosum. Clinical photograph of our patient taken prior to the treatment showing characteristic erythematous subcutaneous nodules around the knees.
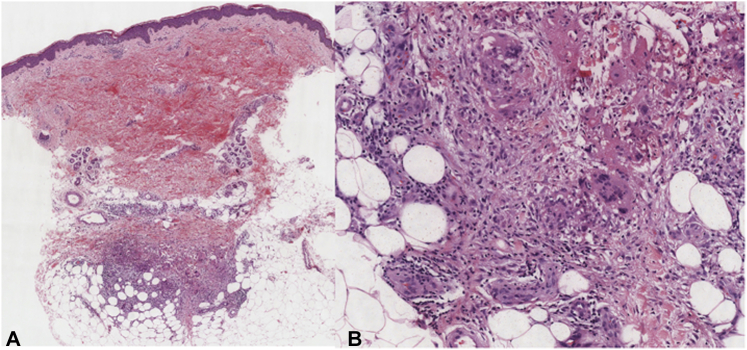
Fig 2.
Histomicrograph depicting erythema nodosum. A, A scanning magnification of the skin biopsy sample from our patient depicts the presence of septal panniculitis. B, A closer magnification of the histomicrograph shows that the subcuticular septa are expanded by an infiltrate composed of epithelioid histiocytes with aggregates of foreign body–type multinucleated giant cells, admixed with Miescher radial granulomas. (A and B, Hematoxylin-eosin stain; original magnifications: A, ×20; B, ×200.)
She was diagnosed with EN triggered by tozinameran vaccination. Complete resolution of her lesions was achieved with colchicine 500 μg twice daily for 1 month. EN is the commonest cause of panniculitis. Although EN can be idiopathic, drug- and vaccine-induced EN is well recognized, aside from other causes due to systemic diseases and infections.
The close temporal relationship between the onset of symptoms and the administration of the tozinameran vaccine is compatible, with the latter being the etiologic agent for our patient's presentation. Other common causes of EN were unlikely, given the lack of other systemic stigmata and complete resolution without relapse. Although she had a previous history of PTB-associated EN, there was no clinico-radiologic evidence of PTB reactivation. The rapid manifestation can be explained by immune priming following the first dose of the vaccination with subsequent vaccine-induced immune upregulation, leading to reactivation of asymptomatic foci of EN that have been dormant since her prior episode.
We also suspect that our patient has a genetic predisposition to EN, given the disease recurrence. EN has been associated with polymorphism of tumor necrosis factor-alfa (TNF-⍺),1 interleukin 1, and interleukin 6 promoter genes. Dysregulation of TNF-⍺ is of particular interest in this patient. The vaccine candidate BNT162b1 was shown to induce a strong TNF-⍺ response,2 and TNF-⍺ also plays a key role in granulomatous diseases such as tuberculosis.3 A polymorphism in the TNF-⍺ promoter gene could account for the development of PTB-associated EN in our patient 10 years ago and now, following COVID-19 vaccination.
Another hypothesis is that this postvaccination phenomenon may be related to autoimmune/autoinflammatory syndrome induced by adjuvants. Panniculitis arising from autoimmune/autoinflammatory syndrome induced by adjuvants has been described before.4 Although the postvaccination phenomena associated with autoimmune/autoinflammatory syndrome induced by adjuvants are typically attributed to aluminum-based adjuvants,5 not much is known about the interactions between our immune systems and the messenger RNA molecule and their lipid carrier particles. This speculative biologic link needs further clarification.
There is currently a paucity of literature on EN caused by messenger RNA COVID-19 vaccines. We hope to raise awareness of tozinameran vaccination as a possible cause of EN by highlighting this case, especially in genetically susceptible patients.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Key words: BNT162b2; COVID-19; drug eruption; erythema nodosum; Pfizer-BioNTech; vaccine.
References
- 1.Labunski S., Posern G., Ludwig S., Kundt G., Bröcker E.B., Kunz M. Tumour necrosis factor-alpha promoter polymorphism in erythema nodosum. Acta Derm Venereol. 2001;81(1):18–21. doi: 10.1080/00015550116912. [DOI] [PubMed] [Google Scholar]
- 2.Sahin U, Muik A, Derhovanessian E, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594-599. Published correction appears in Nature. 2021;590(7844):E17. 10.1038/s41586-020-2814-7 [DOI] [PubMed]
- 3.Mootoo A., Stylianou E., Arias M.A., Reljic R. TNF-alpha in tuberculosis: a cytokine with a split personality. Inflamm Allergy Drug Targets. 2009;8(1):53–62. doi: 10.2174/187152809787582543. [DOI] [PubMed] [Google Scholar]
- 4.Egorova O., Datsina A., Potapova A. POS0422 Panniculitis as a manifestation of an autoimmune/autoinflammatory disease. Ann Rheum Dis. 2021;80:440. doi: 10.1136/annrheumdis-2021-eular.860. [DOI] [Google Scholar]
- 5.Watad A., Sharif K., Shoenfeld Y. The ASIA syndrome: basic concepts. Mediterr J Rheumatol. 2017;28(2):64–69. doi: 10.31138/mjr.28.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]