Fig. 4: CcsBA-closed and -open structures which suggest a mechanism of periplasmic domain movement.
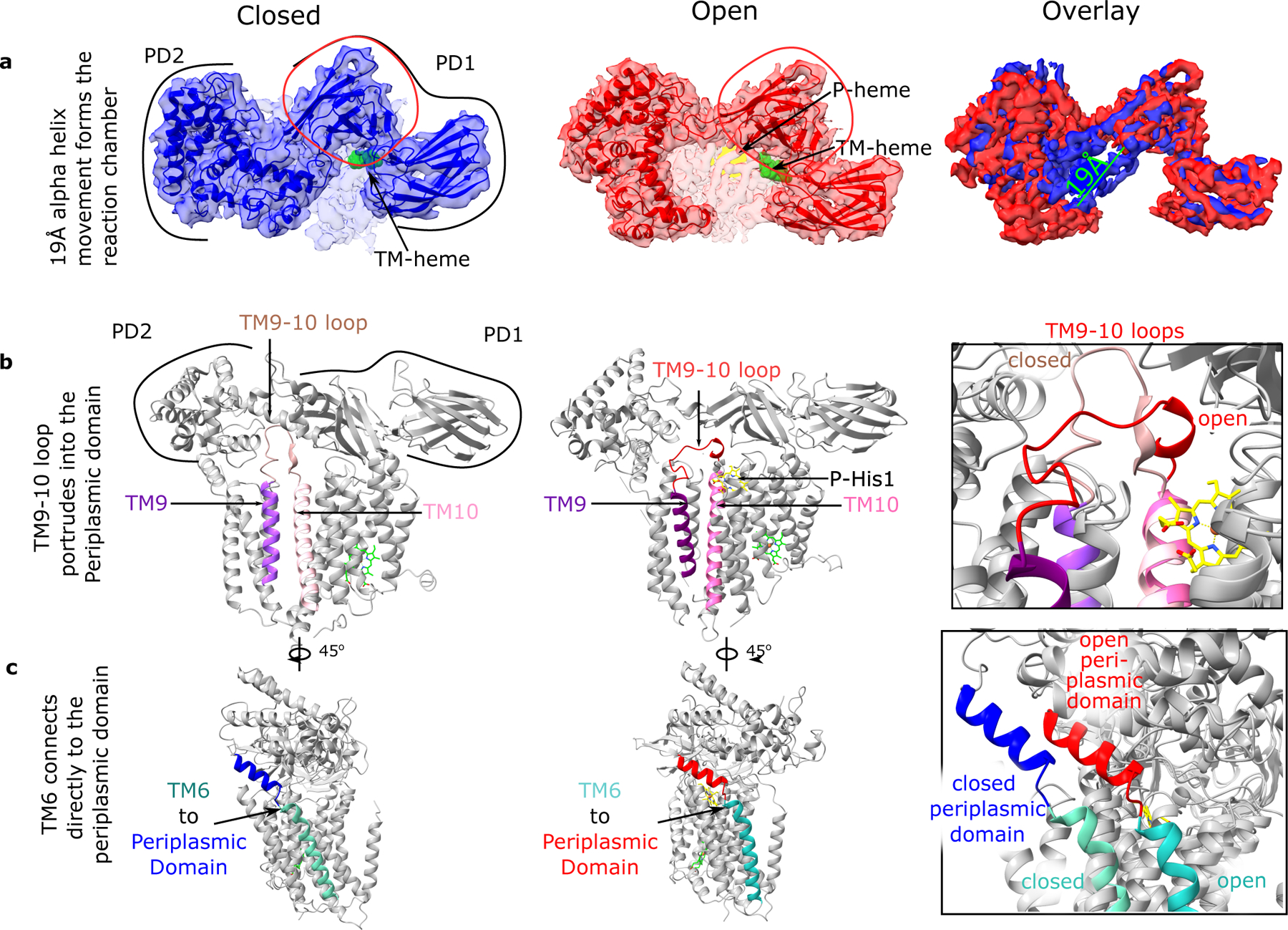
a. Top view of 4.14 Å CcsBA-closed and 3.56 Å CcsBA-open cryo-EM maps and the respective models and their overlay (rightmost panel). P-heme is yellow, and TM-heme is green. Measurement of the periplasmic alpha helices 19 Å movement to open the door to the active site is shown. The red circle encloses the most conserved set of residues in the periplasmic domain. b. Model of CcsBA-closed with the periplasmic domain backbone showing the TM9 to TM10 loop (brown) extending deeply into the periplasmic domain. In contrast, CcsBA-open has the TM9 to TM10 loop (red) extended near the membrane surface. The periplasmic domain was selectively made transparent to see the loops in each panel. c. Model of CcsBA-closed and -open showing the connection of TM6 to the periplasmic domain.
