Extended Data Fig. 1. Topological map of CcsBA and proposed method of apocytc heme attachment.
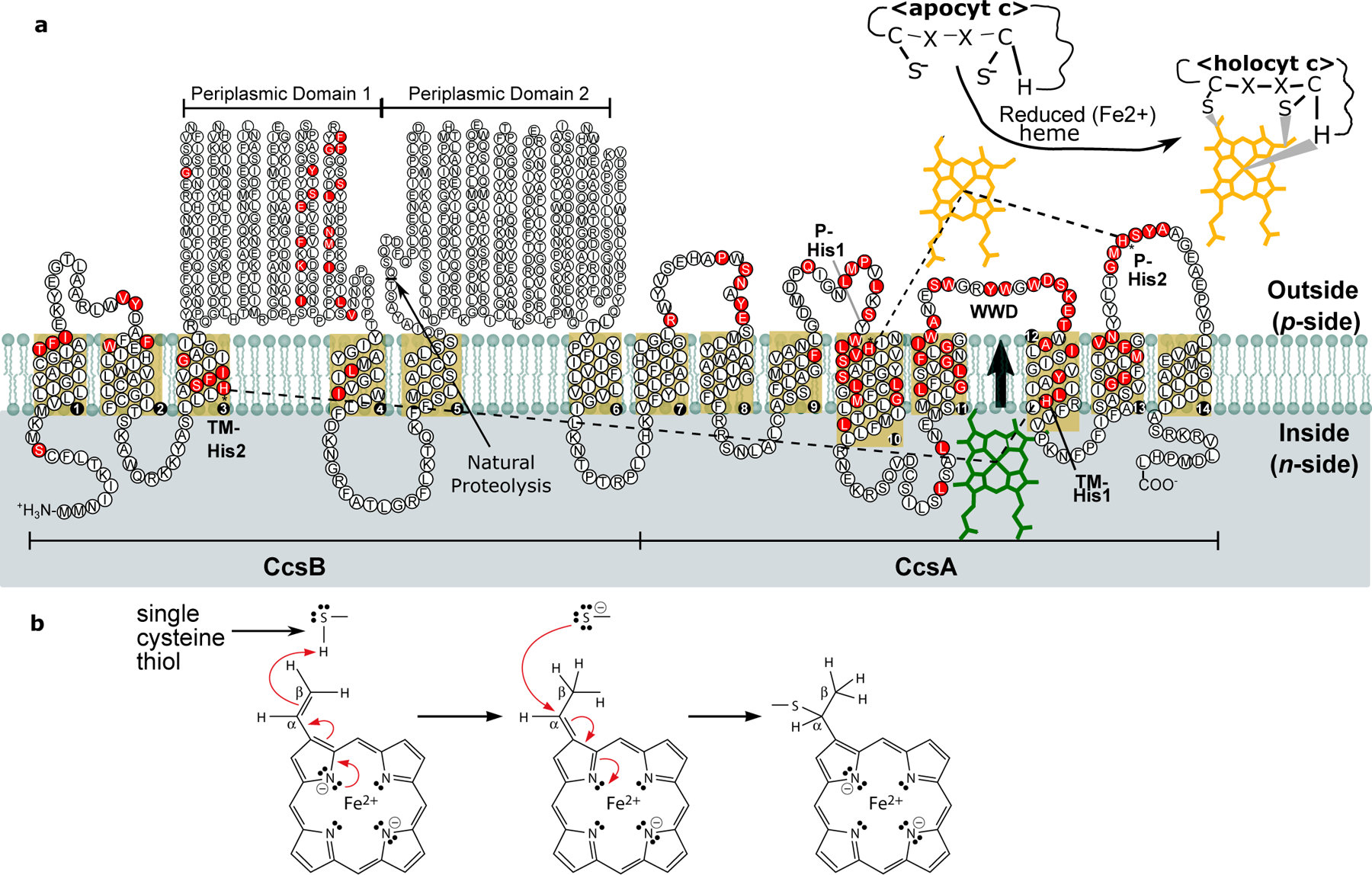
a. Schematic of H. hepaticus CcsBA topology catalyzing heme attachment to apocytc. CcsBA consists of fourteen transmembrane domains and two major periplasmic domains. Conserved features are shown: two conserved histidines in the transmembrane domain (TM-His1-H858, TM-His2-H83) and two conserved periplasmic histidines (P-His1-H897, P-His2-H761) which flank the heme-handling WWD domain. The WWD domain positions heme for attachment to the CXXCH motif in apocytochrome c to form holocytc. Heme enters through a vestibule and is liganded by the TM-His1 and TM-His2. Exact, conserved substitutions and semi-conserved substitutions are all colored in red (T-Coffee analysis1) derived from comparing organisms: M. tuberculosis, B. pertussis, Synechocystis, B. theta, B. subtilis, Wolinella, and H. hepaticus. b. Chemistry of thioether formation. Modified from3. Red arrows indicate two electron transfer.
