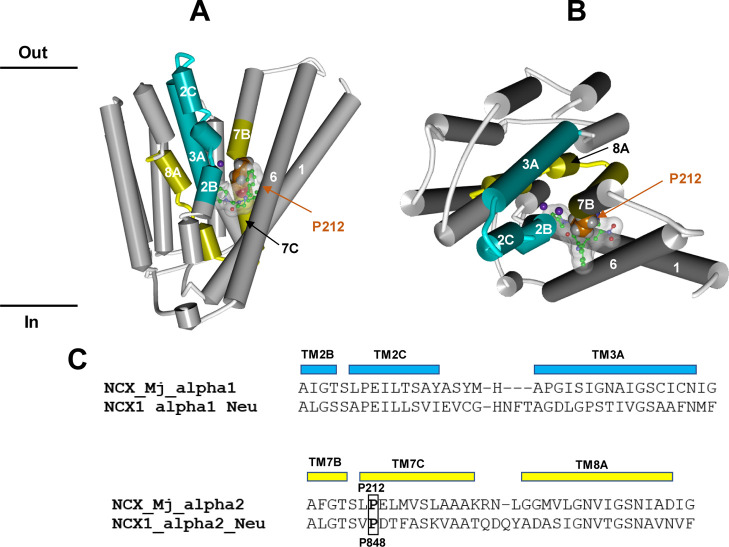
Figure 10.
(A,B). Molecular interaction model between Neurounina-1 and NCX_Mj resulting from our bioinformatic and structural analysis. The X-ray structure of the NCX_Mj transporter in the sodium-loaded semi-open conformation (PDB ID: 5HWY) is colored in white with NCX1_alpha1_Neu and NCX1_alpha2_Neu evidenced in cyan and yellow, respectively. The protein structure is displayed as follows: helical structures as wide cylinders, β-sheets as arrows, and coil and turn regions as tubes. The sodium atoms are displayed in ball and stick and colored in violet. The putative bioactive conformer of Neurounina-1 is displayed in ball and stick and colored by atoms (C = green, O = red, and N = blue). Neurounina-1 solvent accessible surface is showed and colored in white/transparent. Proline P212 is evidenced in CPK and colored in orange. (C) Sequence alignments of the α1 and α2 repeat regions of human NCX1 suggested to be involved in Neurounina-1 binding with the corresponding segments of NCX_Mj.31 NCX_Mj P212 and NCX1 P848 proline residues are evidenced and labeled.