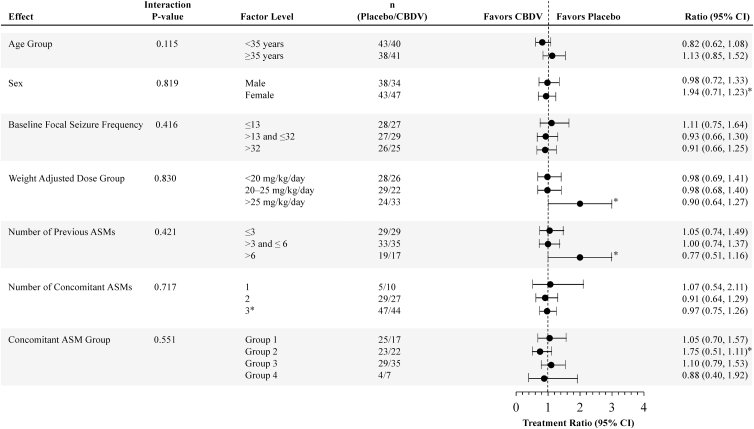
FIG. 2.
Effect modifier analysis of primary efficacy: negative binomial regression effect modifier analysis of focal seizure count during baseline (per 28 days) and treatment (ITT analysis set). Concomitant ASM groups are as follows: Group 1: participants on inducer ASMs (and not on inhibitor ASMs); Group 2: participants on inhibitor ASMs (and not on inducer ASMs); Group 3: participants on ASMs that are neither inducer nor inhibitors; Group 4: patients on inducer and inhibitor ASMs. ASMs, antiseizure medications; CI, confidence interval; N/A, not applicable.
*Correction added on April 15, 2021 after first online publication of February 13, 2021: In Fig. 2, (i) the ratio text values under Ratio (95% CI) for Sex factor level Female was incorrect as 1.94 and should be 0.94 and the ratio text values under Ratio (95% CI) for Concomitant ASM Group factor level Group 2 was incorrect as 1.75 and should be 0.75; (ii) the forest plot data points were incorrect for Number of previous ASMs factor level >6 and Weight Adjusted Dose Group factor level >25 mg/kg/day and have been revised; and (iii) in the Factor Level column, the Number of Concomitant ASMs factor level 3 was incorrect and should be ≥3. The figure has been corrected to reflect the correct values.