Abstract
Background
Youth with bipolar disorder (BP) exhibit poor medication adherence, contributing to affective recurrence. Brief Motivational Interventions (BMIs) improve adherence among adolescents with chronic conditions.
Methods
In an open pilot series, we developed a 3-session BMI for BP adolescents targeting medication adherence and conducted a pilot randomized trial comparing Standard Care (SC) versus SC+BMI. Participants include 43 adolescents with BP prescribed psychotropic medications. We assessed medication adherence objectively via bluetooth-enabled electronic pillbox (MedTracker). A blinded evaluator assessed mood symptoms at intake, 3- and 6-months.
Results
The BMI was well-received. Average objective medication adherence increased with time in SC+BMI, but decreased in SC-Alone (p<0.0001). Adolescents’ baseline self-rated expectation of improvement with treatment moderated the effect of treatment on improvement in adherence over time (p=0.003). Across groups, poor adherence predicted increased likelihood of depression and hypo/mania symptoms in the subsequent two weeks; medication adherence mediated the effect of the BMI on the likelihood of depressive symptoms (p=0.007).
Limitations
Electronic pillbox use (across groups) may enhance adherence, resulting in overestimates compared with naturalistic conditions. This pilot randomized trial may have been underpowered to detect some group differences.
Conclusions
A BMI offers promise as a disseminable adjunctive intervention for improving medication adherence for adolescents with BP. Future studies with larger samples can establish efficacy.
Keywords: medication adherence, brief intervention, adolescent, bipolar disorder
Introduction
Research suggests the mean prevalence rate of bipolar disorder (BP) among adolescents is 2% (Van Meter et al., 2011). Early-onset BP is particularly concerning from a public health standpoint due to its association with poor psychosocial functioning and elevated risk for deleterious outcomes including suicide, substance abuse and legal problems(Pavuluri et al., 2006). Evidence-based treatment guidelines for pediatric BP indicate pharmacotherapy is an essential component of optimal treatment (Findling, 2016) based on randomized studies demonstrating efficacy of several medications (e.g., second generation antipsychotics, lithium, divalpoex) for the treatment of early-onset BP (Goldstein et al., 2017). In clinical practice, pharmacotherapy for pediatric BP commonly involves multiple medications and complex medication regimens (Kowatch et al., 2013). Unfortunately, most youth with BP remain symptomatic and experience recurrences even with adequately prescribed pharmacotherapy (Stepanova and Findling, 2017).
One explanation for the finding that many youth with BP continue to be symptomatic despite adequate treatment is poor medication adherence. Individuals treated for BP rank among the least treatment adherent of all psychiatric populations; on average, 40–50% of patients with BP do not regularly take their medications (Berk et al., 2010). Unique developmental factors including the drives for autonomy and peer acceptance, and intolerance of cosmetic side effects render adolescents especially vulnerable to poor adherence (Taddeo et al., 2008). It is therefore not surprising that studies indicate medication adherence among adolescents with BP is particularly abysmal (DelBello et al., 2007). In a study of pediatric psychiatric inpatients, the most potent predictor of non-adherence post-discharge was a mood disorder diagnosis (Yazdi et al., 2008). Prior studies document non-adherence rates of 44–66% among youth with BP per subjective (self-, parent- and clinician-) report (Coletti et al., 2005; DelBello et al., 2007; Patel et al., 2005). In a recent naturalistic study, our group documented that adolescents with BP did not take over 40% of doses (58% of days) as prescribed over 3 months per objective data (electronic pillbox); furthermore, adolescent-, parent- and clinician-report significantly overestimated medication adherence (Goldstein et al., 2016).
Poor treatment adherence among youth with BP is associated with negative outcomes including higher rates of relapse, longer time to recovery (Gearing and Mian, 2005; Gearing et al., 2009), more frequent and longer hospitalizations (Scott and Pope, 2002), greater psychosocial impairment, substance abuse (Brent and Lerner, 1994) and risk for suicide (Muller-Oerlinghausen et al., 1996). Thus, experts have identified a desperate need for individualized interventions targeting medication adherence for adolescents with serious psychiatric disorders like BP (Colom et al., 2000). Several intensive psychosocial interventions targeting multiple illness-related outcomes for youth with BP have empirical support (Goldstein et al., 2017), yet none has a central focus on promoting adherence. In fact, publications to date on these interventions do not report outcomes related to medication adherence. Among adults with BP, some psychosocial approaches have demonstrated improvements in medication adherence, whereas others have found no effect (Gaudiano et al., 2008). The interventions with support among adults are intensive (5–52 sessions), and largely rely on psychoeducation and cognitive approaches to improving adherence—methods that may not be developmentally appropriate for youth since formal reasoning capabilities that impact medication-taking behaviors are not fully developed until late adolescence/early adulthood (Strait et al., 2012).
Thus, a flexible, client-centered approach to improving treatment adherence may hold greatest potential for benefit (Velligan et al., 2009). Motivational Interviewing (MI), “a collaborative, goal-oriented style of communication with particular attention to language of change, designed to strengthen personal motivation for and commitment to a specific goal by eliciting and exploring the person’s own reasons for change within an atmosphere of acceptance and compassion (Miller and Rollnick, 2013, p.29)” is well-suited for adolescents given the emerging developmental need for autonomy (Naar-King and Suarez, 2011). Evidence supports the use of Brief Motivational Interventions (BMIs) utilizing a MI approach for improving treatment adherence among adolescents with chronic medical conditions such as asthma, diabetes, and HIV (Schaefer and Kavookjian, 2017). Even one session of MI has demonstrated benefit for reducing adolescent health risk behaviors including alcohol use (Newton et al., 2018), and meta-analysis indicates effects are sustained over time (Jensen et al., 2011). Despite its widespread application for adolescents with health risk behaviors and chronic medical conditions, and its exploration among adults with schizophrenia (Drymalski et al., 2009) and depression (Interian et al., 2010), a BMI has not been examined to enhance medication adherence among adolescents with psychiatric disorders.
We thus developed a manualized BMI targeting medication adherence for adolescents with BP and examined its feasibility, acceptability and preliminary efficacy in a pilot randomized trial comparing Standard Care pharmacotherapy (SC) at a pediatric specialty clinic versus SC augmented with the BMI for adolescents with BP. We hypothesized that adolescents receiving SC+BMI would demonstrate greater improvement in objectively assessed medication adherence over 6 months. We also expected that clinician-rated mood symptom severity over follow-up would be mediated by objectively assessed medication adherence in both groups. Improving adherence may interrupt the cycle of poor outcome, minimize long-term debilitating effects of the illness, and lessen costs. Furthermore, an effective adherence–promoting intervention with this population could be applied for adolescents with other psychiatric disorders.
Methods
Treatment Development
We first drafted a treatment manual, and iteratively refined our approach based on clinical experience delivering the intervention for an open case series (n=7). The result is a 3-session BMI (three 30-minute sessions) designed to be conducted in 2 sessions over 4 weeks of treatment, with 1 booster session at month 3. This design overlaps with pharmacological management visit schedules common in clinical settings. Eligible youth are prescribed at least one medication for the management of BP, regardless of current adherence and length of time in treatment.
A Brief Motivational Intervention Delivered Within a Motivational Interviewing Framework.
The BMI is delivered within a Motivational Interviewing (MI) framework {Miller, 2013 #16607}that emphasizes a client-centered, empathic counseling style that: 1) acknowledges, examines and attempts to resolve patient ambivalence to facilitate behavior change; 2) promotes self-efficacy; 3) allows flexibility in strategies for enhancing adherence; 4) reduces discord in the therapeutic relationship and 5) provides individualized feedback to generate greater problem recognition and cognitive reappraisal. The collaborative nature of MI, often referred to as the “spirit” of MI is central to the BMI. MI skills (i.e., person-centered skills, open-ended questions, affirmative statements, reflective listening, summarization) and strategies (elicit and reinforce change talk, soften sustain talk, address and resolve ambivalence, develop a change plan and consolidate commitment; {Miller, 2013 #16607}) are utilized to deliver the BMI components.
Parental Involvement.
MI aims to enhance sense of personal responsibility and control, and regards the patient as the expert regarding behavior change (Dilallo and Weiss, 2009). In keeping with other BMIs for adolescent health behavior change, this BMI incorporates parents in ways that foster behavior change and balance the adolescent’s need for autonomy (Dilallo and Weiss, 2009). As such, the model allows for flexibility surrounding the extent to which parents are involved, based on specific developmental considerations including age, developmental level, independence, family environment, and family’s arrangements regarding dispensing medications.
Core Treatment Components.
The intervention consists of four core treatment components: 1) Eliciting Thoughts and Feelings about Medication and Providing Psychoeducation, 2) Assessing Readiness for Change and Exploring Ambivalence, 3) Creating an Adherence Plan of Action, 4) Evaluating the Adherence Plan of Action (Table 1). The components are designed to be delivered flexibly; because the intervention is person-centered, the BMI progresses differentially between individuals. Session-by-session content is thus suggested rather than prescribed. The aim is to balance the “spirit” of MI with delivery of the core treatment components.
Table 1.
The Four Core Treatment Components of the Brief Motivational Intervention
| Component | Component Content |
|---|---|
| Component 1. Eliciting Thoughts and Feelings about Medication and Providing Psychoeducation |
The clinician invites the adolescent to discuss thoughts and feelings about the role of medication in his/her treatment regimen and his/her BP. This conversation may include inviting the adolescent to share prior patterns of adherence, and feelings about the BP diagnosis and taking medications to stabilize mood symptoms. Based on the adolescent’s information needs and with permission, the clinician then provides personalized psychoeducation regarding the role of medication in the treatment of BP, including discussion of specific symptoms the medication targets, side effects, and potential consequences of both adherence and non-adherence. The clinician then initiates a discussion to identify factors that contribute to adherence for that individual (“What concerns do you have about taking medicine for your BP?”). |
| Component 2. Assessing Readiness for Change and Exploring Ambivalence |
The clinician assesses readiness for change regarding medication adherence. This discussion may include pros and cons of choosing to take medication regularly versus choosing not to. The clinician then encourages the adolescent to explore ambivalence regarding change--what would happen if he/she decided not to make any changes related to taking medication regularly? The clinician also explores the components of readiness for change (i.e., Importance-the desire for change, and Confidence-the assessment of ability to affect change) using the 1–10 readiness ruler as in standard MI practice.34 The clinician highlights and explores any discrepancies between the adolescent’s goals and current medication-taking behaviors. |
| Component 3. Creating an Adherence Plan of Action |
When the adolescent is ready to consider change (consider Importance/Confidence ratings), the clinician and adolescent collaborate to create an individualized adherence plan of action. An ideal adherence plan is detailed, specific, and feasible. The clinician invites the adolescent to generate ideas about how to take medicine more regularly. Specific individualized recommendations are then discussed. If the family has difficulty generating elements of the plan, the clinician provides options [e.g., reminder strategies (e.g., cell phone alarms, text messages), communication strategies for sharing concerns/questions with the treatment team (e.g., write them down before your appointment), and negotiation strategies to assist in establishing developmentally appropriate responsibility for taking medications (e.g., mom places pills in the pill box weekly and adolescent is responsible for taking pill each morning; note taped to front door) and (if necessary) school 35]. The plan is presented to parents, and parental input is encouraged. The clinician and adolescent explore potential barriers and troubleshoot. |
| Component 4. Evaluating the Adherence Plan of Action |
The clinician invites the adolescent to share experiences with medication-taking, as well as use of the adherence plan. The clinician affirms attempts at adherent behaviors, and directly links these attempts to the individual’s goals. The clinician then works with the adolescent to identify and address barriers to implementing the plan. Modifications to the plan are incorporated as needed. The clinician provides encouragement and support to enhance motivation. |
Pilot Randomized Study
Participants.
We recruited participants from the Child and Adolescent Bipolar Services (CABS) specialty clinic at Western Psychiatric Hospital at the University of Pittsburgh Medical Center. Members of the CABS treatment team (psychiatrist, nurse, therapist) approached eligible participants regarding their interest in study participation.
Inclusion Criteria.
Eligible individuals met the following criteria: 1) age 12–22 years; 2) a DSM-IV diagnosis of BPI, II or Not Otherwise Specified (NOS; see Diagnostic Evaluation) via semi-structured interview; 3) no evidence of pervasive developmental disorder; 4) English language fluency and minimum 3rd grade literacy level; 5) engaged in medication management services at CABS and prescribed at least one psychotropic medication for management of BP; 6) able and willing to give informed consent/assent.
Procedures
The University of Pittsburgh Institutional Review Board approved all study procedures, in accord with the Declaration of Helsinki. Study staff explained all procedures to interested adolescents and parents (for participants <18), who provided informed consent for all study procedures.
Diagnostic evaluation.
Trained staff with a master’s degree in a mental health field conducted the diagnostic evaluations. After consent/assent were obtained, evaluators assessed for psychiatric disorders using the semi-structured Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (KSADS-PL; Kaufman et al., 1997) via interview with the adolescent, and for adolescents <18, with the parent about their child. We replaced the KSADS affective disorders module with the KSADS Depression and Mania Rating Scales (DRS, MRS; Axelson et al., 2003) to provide more detailed data on affective symptoms. We applied research-operationalized criteria to diagnose BP NOS (Birmaher et al., 2006; Axelson et al., 2006). KSADS symptom ratings and diagnoses were based on consensus ratings incorporating all available data. Following the evaluator’s assessment, a study-affiliated child psychiatrist conducted a clinical interview with the adolescent and (where applicable) parent. The evaluator and the attending then conferred on the adolescent’s final diagnoses. Inter-rater reliability between the first author and the evaluators for presence/absence of KSADS-PL psychiatric disorders was ≥0.8 (kappa). Inter-rater reliability for KSADS mood items was good (DRS ICC=0.84; MRS ICC=0.96).
Demographics.
Basic demographic information was obtained at study intake using a local demographics form. Socioeconomic status (SES) was ascertained using the Hollingshead four-factor criteria (Hollingshead, 1975).
Randomization.
Participants were randomly assigned to receive either SC alone or SC+BMI (see Figure 1).
Figure 1.
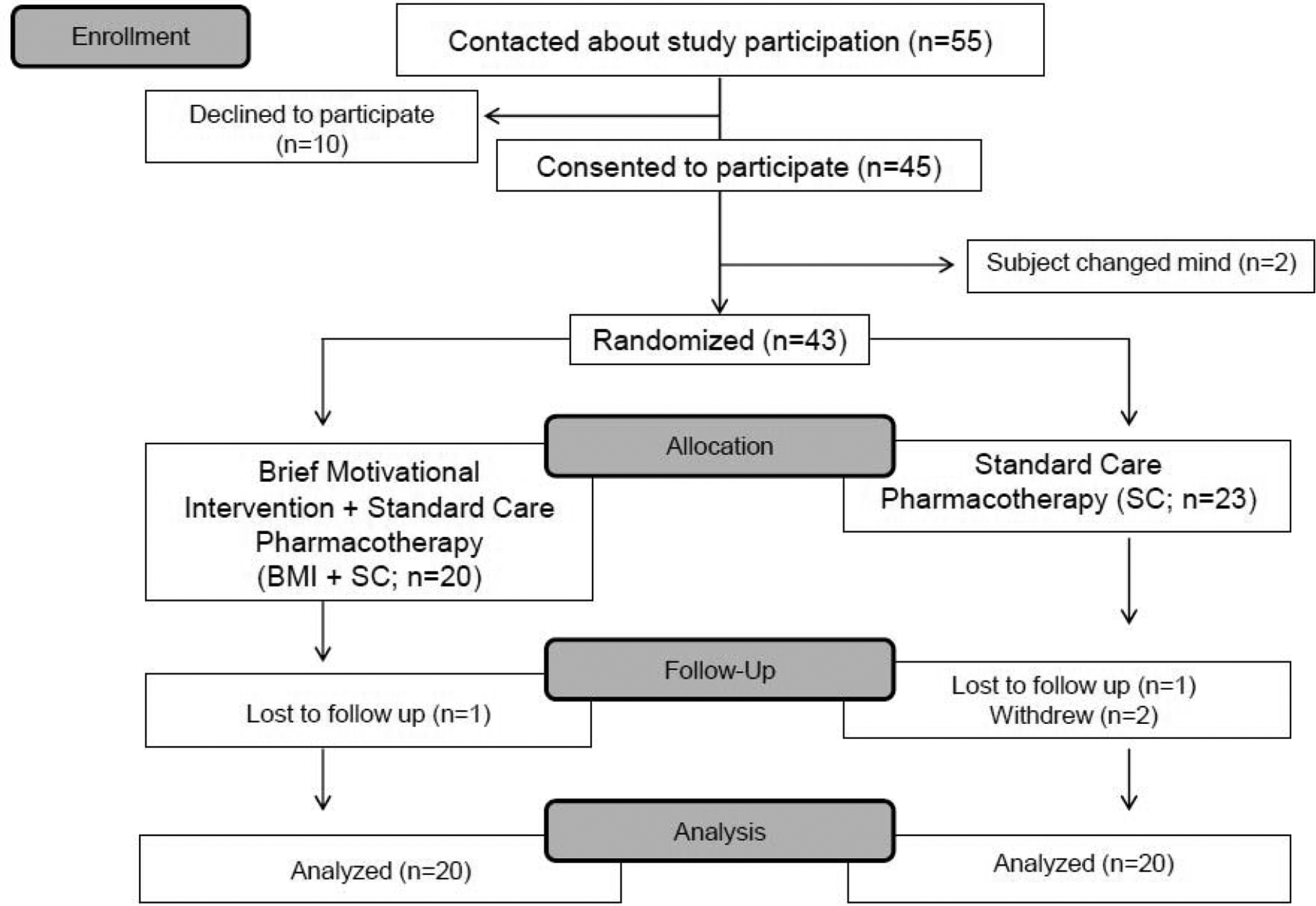
Study CONSORT diagram
Standard Care (SC) Pharmacotherapy.
All study participants received SC pharmacotherapy with a study-affiliated child psychiatrist. SC pharmacotherapy consisted of an initial assessment followed by weekly to biweekly visits for the first month of treatment. Assuming adequate treatment response, visits were scheduled monthly thereafter. When patients were actively symptomatic, appointments were scheduled more frequently. Study psychiatrists managed participants’ medications according to treatment guidelines established by the American Academy of Child and Adolescent Psychiatry for management of pediatric BP (Kowatch et al., 2005). Study psychiatrists reviewed medication decisions in weekly meetings.
BMI Sessions.
Two master’s level mental health professionals with experience conducting therapy with adolescents and their families delivered the BMI. Both study therapists attended initial MI training with a study co-Investigator (AD) certified by the MI Network of Trainers (MINT). MI training respected MINT standards (www.motivationalinterviewing.org) and consisted of didactic instruction and role play to establish a knowledge base in MI principles, skills and strategies. Study-specific training with the first author focused on manual review. Weekly group supervision for study clinicians involved videotape review and discussion of session content.
Treatment Integrity.
Two trained MI adherence coders reviewed videotapes of 20 randomly selected BMI sessions (10 from each study clinician) and provided treatment integrity ratings using the Motivational Interviewing Treatment Integrity Coding protocol (MITI 3.1.1; Moyers et al., 2010). Twenty-minute segments for coding were randomly selected for each session by using a random number generator to select the start time of the coded segment (number randomly selected between 0 and total session minutes minus 20). The MITI provides both a treatment integrity measure for MI clinical trials, and a basis for clinical feedback. Applying standards for measurement of MI fidelity in controlled trials (Jelsam et al., 2015), all mean MITI global score ratings (Global Spirit=4.4±0.5; Evocation=4.2±0.6; Collaboration=4.4±0.8; Autonomy/Support=4.6±0.5; Direction=4.0±0.7; Empathy=4.5±0.7) met or exceeded thresholds for MI “Competence” (i.e., 4.0) {Moyers, 2010 #16609}. With respect to mean MITI behavior count ratings, Percent Complex Reflections=64.9±23.2 exceeded the competence threshold (i.e., 50%) and Percent Open Questions=65.2±11.8 exceeded the Beginning Proficiency threshold (i.e., 50%), whereas the Reflection to Question Ratio (0.82±0.44) and Percent MI-Adherent (80.5±26.4) fell just below Beginning Proficiency cutoffs (i.e., 1.0 and 90% respectively)..Deviations from the protocol were discussed in supervision. Interrater reliability, calculated on 5 videotaped sessions coded by both adherence coders was good (Global item agreement=92%; MI adherent behavior count Pearson’s r=0.9; MI non-adherent behavior count r=0.8).
Instruments
Participants and parents completed assessments with trained study evaluators at intake to determine the subject’s eligibility and establish pre-treatment ratings; eligible participants repeated this battery at 3-months and post-treatment (6-months) with a study evaluator blind to treatment condition. Variables were assessed in the following domains:
Medication Adherence
Objective Adherence.
Upon study entry, each participant received a MedTracker (Hayes et al., 2006), an electronic pillbox for continuous medication adherence monitoring. The MedTracker is a multi-compartment portable pillbox for holding medication, similar to those widely available at pharmacies. The MedTracker records the time the user opens and closes a compartment door via a plunger depressed by the lid upon opening that contacts a switch under the box upon closure. The switch sends a signal to the microcontroller that timestamps the data. Bluetooth wireless connectivity sends data from the MedTracker every 2 hours. The MedTracker yields data on the date and time the pillbox was loaded for the week, single door openings (representing pill-taking events) and time of each event. Prior studies indicate the device is highly reliable in the field with varied patient populations. We selected this technology due to its mobility, automatic data collection, and 7-day pillbox interface. Participants were given instructions on MedTracker use.
Operationalized Definitions of Adherence via MedTracker.
In keeping with our prior work (Goldstein et al., 2016), we operationalized objective medication adherence data as follows: An adherent dose was characterized by MedTracker data indicating: the correct MedTracker door (e.g., the MedTracker door labelled “Monday” was opened on a date that was Monday) was opened within the correct dosing timeframe prescribed, operationalized as follows: morning (2:00am-11:59am); afternoon (12:00pm-4:59pm); evening (5:00pm-1:59am). A wrong-time dose was a correct MedTracker door opening outside of the correct dosing timeframe. A wrong-day dose was an incorrect MedTracker door opening, regardless of dosing timeframe. A dose omission was the absence of the correct MedTracker door opening during the timeframe for a prescribed dose.
Subjective Adherence.
Self- and Parent-Report.
Adolescents and parents (as appropriate) provided medication adherence ratings using a standardized visit form completed at each pharmacotherapy visit and research assessment on which they rated frequency of missed doses of prescribed medications since the last visit on a 1–5 scale: 1=almost never missed (<10% of the time); 2=occasionally missed (10–25% of the time); 3=often missed (25–50% of the time); 4=missed most of the time (50–80% of the time); 5=almost always missed (>80% of the time). The form also assesses for a variety of medication side effects (e.g., headaches, stomachaches).
Physician-Report.
The participant’s psychiatrist completed a standardized Physician’s Visit Form at each pharmacotherapy visit on which they rated their impression of the patient’s medication adherence since the last appointment using the 1–5 scale presented above.
Evaluator Ratings.
The Adolescent Longitudinal Interval Follow-up Evaluation (A-LIFE; Keller et al., 1987) is a semi-structured interview that elicits week-by-week ratings of medication type, dosage, and adherence for all psychotropic drugs prescribed (Leon et al., 2003).
Mood symptoms.
Psychiatric symptoms were monitored at baseline, 3 and 6 months using the A-LIFE psychiatric status ratings (PSR; Keller et al., 1987)) assigned on a 1–6 severity scale (1=no symptoms, 2–4=increasing levels of subthreshold DSM-IV symptoms and impairment, 5–6=full threshold DSM-IV criteria) for mania, hypomania, depression, psychosis, and comorbid disorders for which the subject met criteria at baseline.
Motivational Stages of Change.
The clinician administered an adapted version of the semi-structured Motivational Stages of Change for Adolescents (Gusella et al., 2003) at each BMI session—a developmentally sensitive, reliable and valid measure used to inform individualization of BMI sessions. The measure was originally developed for adolescents with eating disorders and adapted by our group for adolescents prescribed medication for a mood disorder.
Treatment Expectancy.
Adolescents randomized to receive BMI+SC completed the Credibility/Expectancy Questionnaire (Devilly and Borkovec, 2000) at baseline, a psychometrically sound self-report scale for measuring expectancy for improvement in clinical studies.
Treatment Satisfaction.
Following completion of the treatment protocol, participants and parents completed a Treatment Satisfaction Questionnaire consisting of 12 items rated on a 7-point Likert scale.
Data Analysis.
Although not directly comparable due to differences in methods and target population, previous studies of BMIs suggest that a medium effect size (Cohen’s d ~ .4-.5) for adherence can be reasonably anticipated among youth with chronic illness. With a sample size of 40 patients and 6 follow-up time points (monthly for 6 months) estimating 20% attrition and standard assumptions about variances (80% of variance in responses is between participants) and covariances (covariance among adjacent timepoints within participants=.30) of terms in the model, we calculated 80% power a priori to detect a moderate effect of group on adherence for tests of size alpha=.05. We examined for baseline group differences in demographic, clinical, and medication variables using chi-squared, Fisher’s exact, Poisson, and t-tests as appropriate. To compare the objective MedTracker data on adherence with the subjective reports of adherence (1–5 scale above), Spearman correlation coefficients were computed between each subjective rating and corresponding MedTracker-measured adherence. To identify factors significantly associated with medication adherence, each recorded MedTracker dose over follow-up was dichotomized (adherent vs. non-adherent) and modeled via mixed logistic regression. Autocorrelation and partial autocorrelation functions were computed to assess the level and nature of autocorrelation between the within-subject repeated observations. Results indicated the covariance pattern of these data was expectedly autoregressive, so an ante-dependent covariance pattern was fitted in all logistic models to account for the autoregressive covariance pattern while also allowing for unequal time-spacing between MedTracker observations and non-constant correlation structure since correlation structure between sequential doses depends on factors such as time of day (Kenward, 1987). All models controlled for potential confounding factors previously identified (Goldstein et al., 2016), including number of daily doses, weekday vs. weekend, dose timing (morning, afternoon, evening), and days since/until the closest clinical assessment. Models further controlled for baseline MedTracker adherence rates as recorded before first treatment session in BMI+SC (median duration = 14 days) or during the first two weeks in the SC group. Primary effects included treatment as well as treatment-by-time and treatment-by-baseline adherence interaction effects. Secondary analyses were performed within the BMI+SC group to test potential moderators of the treatment effect including baseline adherence, length and frequency of BMI sessions, and treatment expectancy. All interaction effects were mean-centered to improve interpretability. Subsequent analyses assessed the direction and proximity of association between weekly adherence rates and PSR measures of hypo/mania and major depression via cross-correlation estimation. Lagged logistic regressions were then fit based upon the cross-correlation results to assess the effect of weekly adherence rate on the likelihood of hypo/manic and depressive symptoms two weeks later (random intercepts were fit to account for within-subject correlation). Lagged regressions controlled for concurrent and next-week mood symptoms to ensure that any estimated effects of adherence were partitioned from the possible effects of lingering subthreshold symptoms from previous weeks. A multilevel path model was then further fit to test whether repeated measures of adherence (by dose) mediated the effect of BMI on the likelihood of hypo/manic and depressive symptoms two weeks after each dose. Multilevel path models were fit using Mplus5; all other analyses were performed using SAS9.4. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Study Sample
Sample demographics and clinical characteristics are presented in Table 2. The sample included 43 youth with BP with a mean age of 16 years. The groups were similar on most demographic and clinical variables examined (p>0.1 for all), with the exception of BP subtype (p=0.03); a trend for baseline depression severity (p=0.08).
Table 2.
Sample Demographic and Clinical Characteristics
| Variable | Standard Care + Brief Motivational Intervention (SC+BMI) (n=20) |
Standard Care Alone (n=23) |
Test Statistic | p-value |
|---|---|---|---|---|
| Age (years) | 16.6 ± 2.4 | 15.7 ± 3.0 | t=1.02 | 0.3 |
| Sex (male) | 7 (35.0%) | 13 (56.5%) | χ2=1.99 | 0.2 |
| Race | Fisher’s Exact Test | 0.4 | ||
| White | 13 (65.0%) | 18 (78.3%) | ||
| African-American | 4 (20.0%) | 4 (17.4%) | ||
| More than one race | 3 (15.0%) | 1 (4.4%) | ||
| SES total score† | 3.1 ± 1.4 | 3.5 ± 1.4 | t=0.88 | 0.4 |
| Living Situation (both biological parents) | 5 (25.0%) | 11 (47.8%) | χ2=2.39 | 0.1 |
| Bipolar Subtype | Fisher’s Exact Test | 0.03 | ||
| BP-I‡ | 2 (10.0%) | 10 (43.48%) | ||
| BP-II | 6 (30.0%) | 2 (8.7%) | ||
| BP-NOS§ | 12 (60.0%) | 11 (47.8%) | ||
| Age of BP onset | 11.4 ± 4.0 | 11.0 ± 4.8 | t=0.24 | 0.8 |
| Baseline Depression Severity¥ | Fisher’s Exact Test | 0.08 | ||
| No Symptoms | 2 (10.0%) | 9 (39.1%) | ||
| Subthreshold Symptoms | 11 (55.0%) | 10 (43.5%) | ||
| Threshold Symptoms | 7 (35.0%) | 4 (17.4%) | ||
| Baseline Hypo/mania Severity | Fisher’s Exact Test | 0.7 | ||
| No Symptoms | 9 (45.0%) | 14 (60.9%) | ||
| Subthreshold Symptoms | 10 (50.0%) | 8 (34.8%) | ||
| Threshold Symptoms | 1 (5.0%) | 1 (4.4%) | ||
| # Current Diagnoses | 2.8 ± 0.8 | 2.5 ± 1.1 | t=1.11 | 0.3 |
Socioeconomic status (Hollingshead Redlich criteria,Hollingshead, 1975)
BP = bipolar disorder
NOS = Not otherwise specified (COBY operationalized criteria, Birmaher et al., 2006; Axelson et al., 2006)
Adolescent Longitudinal Interval Follow-up Evaluation Psychiatric Status Ratings (A-LIFE; Keller et al., 1987)
Medication Information
On average, youth were prescribed 3 (SD=1.1, Range 1–5) psychotropic medications at study intake; 40 (93.0%) were prescribed >1 medication (Table 3). Medication classes prescribed and timing of medication dosing were not significantly different between groups (p>0.1 for all). Over the 6-month study period, 60.5% of participants experienced at least one medication change (i.e., discontinued a medication or prescribed a new medication), and 83.7% experienced at least one dosing change (dose, time of day, number of doses).
Table 3.
Psychotropic Medications Prescribed at Study Intake
| Variable | Standard Care + Brief Motivational Intervention (SC + BMI) (n=20) |
Standard Care Alone (n=23) |
Test Statistic | p-value |
|---|---|---|---|---|
| # Psychotropic medications at intake | 2.7 ± 1.0 | 2.9 ± 1.2 | Wald χ2=0.27 | 0.6 |
| # Daily doses at intake | 3.5 ± 1.5 | 4.0 ± 2.0 | Wald χ2=0.74 | 0.4 |
| Morning Dose | 18 (90.0%) | 20 (87.0%) | Fisher’s Exact | ~1 |
| Afternoon Dose | 5 (25.0%) | 4 (17.4%) | Fisher’s Exact | 0.7 |
| Evening Dose | 16 (80.0%) | 22 (95.7%) | Fisher’s Exact | 0.2 |
| Antipsychotics | 27 (39.1%) | 39 (42.9%) | χ2=0.22 | 0.6 |
| Antidepressants | 8 (11.6%) | 6 (6.6%) | χ2=1.23 | 0.3 |
| Stimulants | 13 (18.8%) | 13 (14.3%) | χ2=0.60 | 0.4 |
| Mood Stabilizers | 16 (23.2%) | 14 (15.4%) | χ2=1.57 | 0.2 |
| Benzodiazepines | 0 (0.0%) | 4 (4.4%) | Fisher’s Exact | 0.1 |
| Other | 5 (7.3%) | 15 (16.5%) | χ2=3.06 | 0.08 |
BMI Sessions
Youth randomized to receive BMI+SC received an average of 2.9 of the 3 planned BMI sessions (Range 2–3). Ratings from youth and parents indicate the frequency of BMI visits was appropriate (1–7 scale where 4=appropriate; youth M=4.0±1.2; parent M=4.000B10), and high satisfaction with the BMI (1–7 scale where 7=very satisfied; youth satisfaction M=6.0±1.2; parent satisfaction M=6.6±1.1).
Medication Adherence
Medication Adherence.
Subjects had a median of 3.5 months of MedTracker data with a median of 198 total doses (treatment groups did not differ in average follow-up length or number of doses; ps>0.4). MedTracker data indicated average adherence approximately 67% in the first month of follow-up, and 60% over the entire follow-up period. Mixed logistic regressions modeling adherence by dose (6,918 total datapoints modeled) estimated that higher baseline MedTracker adherence rates, fewer daily doses, fewer days since/until next clinic visit, and weekdays were associated with greater estimated adherence probability (all ps<0.009). Evening doses were associated with greatest estimated adherence, followed closely by morning doses and distantly by afternoon doses (p<0.0001).
While the two groups did not differ in adherence probability on average (F=0.23, p=0. 63), the treatment-by-time interaction effect was highly significant (F=21.70, p<0.0001) and indicated that average adherence in BMI+SC was estimated to increase by approximately 1% per month, whereas average adherence in SC Alone was estimated to decrease by approximately 5% per month (Figure 2). Further testing found that neither the treatment nor time-slope effects were significantly moderated by baseline MedTracker adherence rates (all ps>0.2). Analyses within the BMI+SC subsample found that length and frequency of BMI sessions did not have a significant effect on estimated adherence as direct effects or as moderators of the treatment effect (all ps>0.2). Baseline expectancy for improvement with treatment significantly moderated the treatment effect such that participants with higher expectation of improvement with treatment exhibited improvement in adherence more quickly than subjects with lower expectation (F=8.79, p=0.003). Motivational stages of change assessed at baseline and during follow-up did not significantly moderate the treatment effect (ps>0.4).
Figure 2.
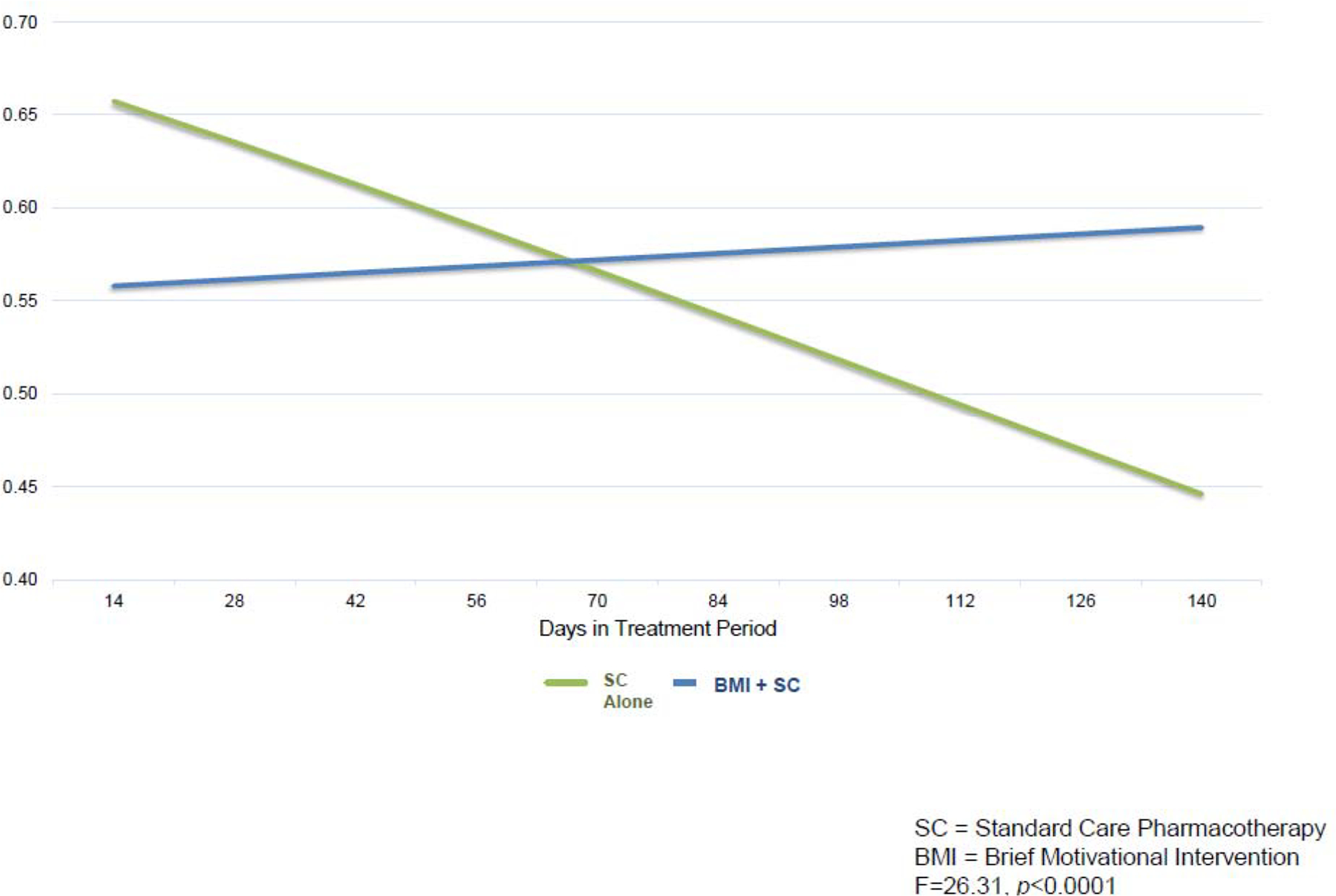
Treatment by time interaction effect on objective medication adherence via MedTracker
Adolescents, parents, and physicians all reported average adherence ratings of “1” (almost never missed) at intake, and average ratings throughout follow-up never fell below “2” (occasionally missed) on the 1–5 scale. Due to lack of variation, statistical analyses of these measures found no associations or group differences. Correlations between concurrent objective and subjective measurements of adherence were low (child-report rS=0.30, parent-report rS=0.29, physician-report: rS=0.49),
Mood Symptom Severity.
Using cross-correlation estimation to assess the direction and proximity of association between weekly adherence rates and PSR ratings of hypo/mania and depression, we found weekly adherence ratings were most strongly correlated with PSR hypo/mania and major depression scores 1–2 weeks later. For this reason, lagged logistic regressions were fit to assess the effect of weekly adherence rate on likelihood of hypo/manic, depressive, or any mood symptoms (at full threshold or subthreshold level) two weeks later. Results indicated that greater weekly adherence rates were significantly associated with reduced likelihood of depressive symptoms (F=7.80, p=0.005) and any mood symptoms (F=5.50, p=0.02) two weeks later, and there was a marginal association with reduced likelihood of hypo/manic symptoms two weeks later (F=3.34, p=0.07). Graphical analysis revealed that while the association between weekly adherence rates and likelihood of hypo/manic symptoms two weeks later was fairly linear, this was not the case for depressive symptoms. Rather, likelihood of depressive symptoms dropped and was fairly constant where weekly adherence was 60% or greater. For this reason, lagged regression was repeated for the depression and any mood outcomes dichotomizing the weekly adherence predictor variable at 60+%. Results indicated that subjects who were ≥60% adherent in a given week were 3.6 times less likely to display depressive symptoms two weeks later (F=9.27, p=0.003) and 2.7 times less likely to display any mood symptoms two weeks later (F=8.23, p=0.004).
Mediational Model.
Lastly, we conducted a multilevel path model to test whether repeated measures of medication adherence (by dose) mediated the effect of BMI on the likelihood of mood symptoms two weeks after each dose (adherence portion of the model controlled for potential confounding dosage factors). Results indicated a highly significant mediational effect on depression (z=2.72, p=0.007), though the effect was nonsignificant on hypo/mania (z=0.32, p=0.8). Results of the adherence portion of the model indicated a significant treatment-by-time interaction effect and significant effects of dosage factors including day of week, timing, and number of doses, as well as days since/until the closest clinical assessment (all ps<0.0001).
Discussion
Despite the availability of newer and more effective psychotropic drugs, this progress is futile if we do not improve poor rates of adherence with prescribed medications in youth with BP. This is the first study to investigate an intervention expressly focused on enhancing medication adherence in this population. A unique strength is the use of an objective measure of adherence throughout the trial. The BMI was well-received by adolescents and their parents. Outcome data indicate that medication adherence improved with time among youth receiving the adjunctive BMI but decreased among youth receiving SC Alone. Furthermore, baseline self-ratings of treatment expectations with the BMI moderated improvement in objectively measured adherence. While poor medication adherence predicted increased likelihood of mood symptoms (both depression and hypo/mania) in the subsequent two weeks in both treatment groups, adherence mediated this effect for depressive symptoms among youth receiving the BMI.
The BMI was expressly designed to be delivered to youth of various age and developmental levels, and flexibly integrated within standard pharmacological management for pediatric BP. The provision of Essential Treatment Ingredients rather than prescribed session content allows the clinician to individualize the pace of the intervention based on the youth’s level of knowledge and readiness for change. These assets may render the BMI a particularly promising approach for youth with chronic psychiatric disorders like BP.
Although MI spirit, skills, and strategies are broadly implemented in brief interventions targeting a range of behaviors, data on fidelity are often lacking (Jelsma et al., 2015). In this study, clinicians delivered the intervention, on the whole, with fidelity to MI. This finding, taken with data indicating that MI can be learned via minimally intensive training experiences by individuals with varied levels of education and experience, and fidelity enhanced with supervision and feedback (Madson et al., 2009), provides support for its ultimate disseminability.
Outcome data indicate that per objective data, medication adherence improved with time among youth receiving the adjunctive BMI but decreased among youth receiving SC Alone. To our knowledge, this is the first psychosocial intervention to demonstrate an impact on medication-taking behavior among youth with BP. Although some psychosocial approaches have demonstrated improvements in medication adherence in adults with BP (Gaudiano et al., 2008), these interventions are more intensive than the BMI. Coupled with its potential efficacy, the BMI’s brevity may be a particularly appealing feature given the treatment burden faced by many youth with BP and their families (Dusetzina et al., 2012). Thus, if these findings are replicated, future work may consider employing principles from dissemination and implementation science to broadly disseminate the BMI. Relatedly, application of the BMI for youth with other chronic psychiatric disorders requiring psychopharmacological management should be considered.
We also demonstrated an association between poor medication adherence and clinician-rated mood symptoms in the subsequent two weeks in both treatment groups. Results indicate that subjects who were ≥60% adherent in a given week were 3.6 times less likely to display depressive symptoms and 2.7 times less likely to display any mood symptoms two weeks later. Critically, adherence mediated the effect of the BMI on likelihood of depressive symptoms 2 weeks later. Through an experimental therapeutics lens, these data provide preliminary support for the BMI’s unique capacity to engage the target mechanism (medication adherence) to affect outcome (mood symptoms).
In keeping with a large body of work demonstrating the potency of treatment expectancy as a predictor of treatment response in evidence-based psychotherapy (Arnkoff, Glass, Shapiro & Norcross, 2002), we found that baseline self-ratings of treatment expectations with the BMI moderated improvement in adherence with the intervention. As such, clinicians may consider assessing youths’ treatment expectations prior to initiating treatment. A subsequent focus on enhancing treatment expectations through such techniques as presentation of a strong treatment rationale (Ahmed & Westra, 2009) may therefore further enhance outcomes.
Subjective reports of adherence by youth, parents and physicians had limited variability over time, and overestimated adherence as compared with objective data. These findings are in keeping with prior medication adherence studies with varied patient populations showing a mean correlation between subjective and objective methods of 0.5 (Riekert and Rand, 2002). Innovations in digital medicine, like the recently FDA-approved antipsychotic pill embedded with a sensor that detects and records pill ingestion (www.abilifymycite.com) may offer substantial improvements in the reliable assessment of medication adherence.
Limitations
This study was a pilot randomized trial, and therefore may have been underpowered to detect some meaningful differences between groups. However, a post-hoc power analysis estimating the minimum detectable effect size given observed group sizes, adherence rates, and average number of MedTracker observations indicates 80% power to detect odds ratios of 1.5, which translates to a small-to-medium effect size. Nonetheless, further testing with larger samples and extended follow-up is needed to establish the BMI’s efficacy. Additionally, this study was conducted at a specialized psychiatric clinic for youth with BP. As such, it is unknown to what extent youth enrolled in the trial may be representative of the larger population of youth with BP. Additionally, rates of BP subtypes differed significantly between groups, so questions remain regarding the intervention’s efficacy among youth across the BP specvtrum. While the BMI affords flexibility regarding extent of parental involvement, we did not systematically collect such data; future studies may consider parental involvement as a mediator of outcome. Our objective measure of medication adherence involved use of an electronic pillbox. Given data indicating that use of such a device may enhance adherence (Costa et al., 2015), it is possible that objective adherence rates described herein overestimate adherence under naturalistic conditions. Yet, since youth in both treatment conditions used the pillbox, any effects would similarly impact both treatment groups. Furthermore, while objective methods (like the Medtracker we employed) are considered the “gold standard” (Cramer, 1995) and provide more detailed and reliable data on adherence behaviors than subjective report (which overestimate adherence as compared with objective data), resultant data do not conclusively demonstrate that the patient ingested the medication (Riekert and Rand, 2002).
An adjunctive BMI may hold promise for enhancing medication adherence, thereby improving outcomes for youth with BP. The developmental appropriateness, acceptability, and potential for widespread dissemination of the model may render this approach widely applicable for youth with a range of psychiatric presentations.
Highlights.
Subjective reports of medication adherence overestimated adherence as compared with objective data.
Objective adherence decreased with time among youth receiving standard care.
Objective adherence improved with time among youth receiving the adjunctive BMI.
Poor objective adherence predicted greater likelihood of subsequent mood symptoms.
Adherence mediated the effect of adherence on depressive symptoms for youth receiving the BMI.
Acknowledgements:
The authors thank Reality Price MA, Matthew Garcia BS, Jamie Feldman, and the faculty and staff of the Child and Adolescent Bipolar Spectrum Services Clinic at the University of Pittsburgh.
Funding: This work was supported by the National Institute of Mental Health (R34 MH092424). The funding source had no role in study design, collection, analysis or interpretation of data; in the report writing; or in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Statement
All authors have approved the manuscript. The manuscript is not under consideration by another publication and has not been previously published in whole or substantial part. If accepted, the manuscript will not be published elsewhere in the same form, in English or in any other language, including electronically, without the written consent of the copyright holder.
Competing Interests
Dr. Goldstein is an employee of the University of Pittsburgh and the University of Pittsburgh Medical Center and receives research funding from the National Institute of Mental Health, the American Foundation for Suicide Prevention, the University of Pittsburgh CTSI, and The Brain and Behavior Foundation. She receives royalties from Guilford Press.
Ms. Krantz has no competing interests to declare.
Ms. Fersch-Podrat is an employee of the University of Pittsburgh Medical Center.
Ms. Hotkowski is an employee of the University of Pittsburgh and the University of Pittsburgh Medical Center.
Mr. Merranko is an employee of the University of Pittsburgh Medical Center.
Dr. Sobel has no competing interests to declare.
Dr. Axelson is an employee of The Ohio State University and receives Royalties from UpToDate and serves as a consultant for Janssen Research.
Dr. Birmaher is an employee of the University of Pittsburgh and the University of Pittsburgh Medical Center, and receives research funding from the National Institute of Mental Health. He receives royalties from Random House, Lipincott Williams and Wilkins, and UpToDate.
Dr. Douaihy is an employee of the University of Pittsburgh and the University of Pittsburgh Medical Center, and receives research funding from the National Institute of Mental Health, the National Institute on Drug Abuse, American Foundation for Suicide Prevention, National Heart, Lung, and Blood Institute (NHLBI), SAMHSA, HRSA, and Alkermes. He receives royalties for academic books from Oxford University Press, Springer, and PESI Publishing & Media.
References
- Ahmed M, Westra HA Impact of a treatment rationale on expectancy and engagement in cognitive behavioral therapy for social anxiety. Cognit Ther Res. 2009;33:314–322. [Google Scholar]
- Angold A, Costello EJ, Messer S, Pickles A, Winder F, Silver D. The development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. Int J Meth Psych Res. 1995;5:237–249. [Google Scholar]
- Arnkoff DB, Glass CR, Shapiro SJ, Norcross JC Psychotherapy relationships that work: Therapist contributions and responsiveness to patients. New York, NY: Oxford University Press; 2002. [Google Scholar]
- Axelson DA, Birmaher B, Brent D, Wassick S, Hoover C, Bridge J, Ryan N. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-age Children Mania Rating Scale for Children and Adolescents. J Child Adol Psychop. 2003;13(4):463–470. [DOI] [PubMed] [Google Scholar]
- Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(10):1139–1148. [DOI] [PubMed] [Google Scholar]
- Berk L, Hallam K, Colom F, Vieta E, Hasty M, Macneil C, Berk M. Enhancing medication adherence in patients with bipolar disorder. Hum Psychopharm. 2010;25:1–16. [DOI] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, et al. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63(2):175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, Lerner MS. Cognitive therapy with affectively ill, suicidal adolescents. In: Wilkes TCR, Belsher G, Rush AJ, Frank E, eds. Cognitive Therapy for Depressed Adolescents New York: The Guilford Press; 1994:298–320. [Google Scholar]
- Coletti DJ, Leigh E, Gallelli KA, Kantafaris V. Patterns of adherence to treatment in adolescents with bipolar disorder. J Child Adol Psychop. 2005;15 (6):913–917. [DOI] [PubMed] [Google Scholar]
- Colom F, Vieta E, Martinez-Aran A, Reinares M, Benabarre A, Gasto C. Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiat. 2000;61 (8):549–555. [DOI] [PubMed] [Google Scholar]
- Costa E, Giardini A, Savin M, Menditto E, Lehane E, Laosa O, Pecorelli S, Monaco A, Marengoni A. Interventional tools to improve medication adherence: review of the literature. Patient Prefer Adher. 2015;9:1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer JA. Microelectronic systems for monitoring and enhancing patient compliance with medication regimens. Drugs. 1995;49:321–327. [DOI] [PubMed] [Google Scholar]
- DelBello MP, Hanseman D, Adler C, Fleck DE, Strakowski SM. Twelve month outcome of adolescents with bipolar disorder following first-hospitalization for a manic or mixed episode. Am J Psychiat. 2007. [DOI] [PubMed] [Google Scholar]
- Devilly G, Borkovec TD. Psychometric properties of the crdibility/expectancy questionnaire. J Behav Ther Exp Psy. 2000;31:73–86. [DOI] [PubMed] [Google Scholar]
- Dilallo J, Weiss G. Motivational interviewing and adolescent psychopharmacology. J Am Acad Child Psy. 2009;48(2):108–113. [DOI] [PubMed] [Google Scholar]
- Drymalski W, Campbell T. A review of motivational interviewing to enhance adherence to antipsychotic medication in patients with schizophrenia: Evidence and recommendations. J Ment Health 2009;18:6–15. [Google Scholar]
- Dusetzina S, Farley J, Weinberger M, Gaynes B, Sleath B, Hansen R. Treatment use and costs among privately insured youths with diagnoses of bipolar disorder. Psychiatr Serv. 2012;63(10):1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL. Evidence-based pharmacologic treatment of pediatric bipolar disorder. J Clin Psychiat. 2016;77(suppl E1). [DOI] [PubMed] [Google Scholar]
- Gaudiano B, Weinstock L, Miller I. Improving treatment adherence in bipolar disorder: A review of current psychosocial treatment efficacy and recommendations for future treatment development. Behav Modif. 2008;32(3):267–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing RE, Mian IA. An approach to maximizing the treatment adherence of children and adolescents with psychotic disorders and major mood disorders. The Canadian Child and Adolescent Psychiatry Review. 2005;14(4):106–113. [PMC free article] [PubMed] [Google Scholar]
- Gearing RE, Mian IA, Sholonsky A, Mian I, Barber J. Developing a risk model of time to first-relapse for children and adolescents with a psychotic disorder. J Nerv Ment Dis. 2009;197(1):6–14. [DOI] [PubMed] [Google Scholar]
- Goldstein TR, Krantz M, Merranko J, Garcia M, Sobel L, Rodriguez C, Douaihy A, Axelson D, Birmaher B. Medication adherence among adolescents with bipolar disorder. J Child Adol Psychop. 2016;26(10):864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Birmaher B, Carlson G, DelBello M, Findling R, Fristad M, Kowatch R, Miklowitz D, Nery F, Perez-Algorta G, Van Meter A, Zeni C, Correll C, Kim H, Wozniak J, Chang K, Hillegers M, Youngstrom E, The International Society for Bipolar Disorders Task Force report on pediatric bipolar disorder: Knowledge to date and directions for future research. Bipolar Disorders. 2017;19(7):524–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella J, Butler G, Noichols L, Bird D. A brief questionnaire to assess readiness to change in adolescents with eating disorders: Its applications to group therapy. Eur Eat Disord Rev. 2003;1(1):58–71. [Google Scholar]
- Hayes T, Hunt J, Adami A, Kaye J. An electronic pillbox for continuous monitoring of medication adherence. Conf Proc IEEE Eng Med Biol Soc. 2006;1:6400–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead A Four Factor Index of Social Status. Unpublished paper: Yale University; 1975. [Google Scholar]
- Interian A, Rios L, Martinez I, Krejci J, Guarnaccia PJ. Adaptation of a motivational interviewing intervention to improve antidepressant adherence among Latinos. Cult Divers Ethn Min. 2010;16(2):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelsma J, Mertens V, Forsberg L, Forsberg L. How to measure Motivational Interviewing fidelity in randomized controlled trials: Practical recommendations. Contemp Clini Trials. 2015;43:93–99. [DOI] [PubMed] [Google Scholar]
- Jensen C, Cushing C, Aylward B, Craig J, Sorell D, Steele RE. Effectiveness of motivational interviewing interventions for adolescent substance use behavior change: a meta-analytic review. J Consult Clin Psych. 2011;79(4):433–440. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Psy. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott P, Andreasen N. The Longitudinal Interval Follow-Up Evaluation: A comprehensive method for outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. [DOI] [PubMed] [Google Scholar]
- Kenward MG. A method for comparing profiles of repeated measurements. Appl Stat. 1987;36:296–308. [Google Scholar]
- Kowatch R, Fristad MA, Birmaher B, Wagner KD, Findling R, Hellander M. Treatment guidelines for children and adolescents with bipolar disorder. J Am Acad Child Psy. 2005;44(3):213–235. [DOI] [PubMed] [Google Scholar]
- Kowatch R, Youngstrom E, Horwitz S, Dememter C, Fristad M, Birmaher B, Axelson D, Ryan N, Frazier T, Arnold E, Young A, Gill MK, Findling R. Prescription of psychiatric medications and polypharmacy in the LAMS cohort Psychiatric Services. 2013;64(10):1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon AC, Solomon DA, Mueller TI, Endicott J, Rice J, Maser J, Coryell W, Keller M. A 20-year longitudinal observational study of somatic antidepressant treatment effectiveness. Am J Psychiat. 2003;160:727–733. [DOI] [PubMed] [Google Scholar]
- Madson M, Loignon AC, Lane C. Training in motivational interviewing: A systematic review. J Subst Abuse Treat. 2009;36:101–109. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. Vol 2. New York: Guilford Press; 2002. [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing: Helping people change. Edition 3. New York, NY: The Guilford Press; 2013. [Google Scholar]
- Muller-Oerlinghausen B, Wolf T, Ahrens B, Glaenz T, Schou M, Grof E, Grof P, Lenz G, Simhandl C, Thau K, Vestgaard P, Wolf R. Mortality of patients who dropped out from regular lithium prophylaxis: A collaborative study by the International Group for the Study of Lithium-Treated Patients (IGSLI). Acta Psychiatr Scand. 1996;94:344–347. [DOI] [PubMed] [Google Scholar]
- Motivational Interviewing Network of Trainers (MINT). www.motivationalinterviewing.org/about_mint. Accessed March 25, 2019.
- Moyers TB, Martin T, Manual JK, Miller WR, Ernst D. Motivational interviewing treatment integrity 3.1.1 (MITI 3.1.1), 2010, Center on Alcoholism, Substance Abuse and Addictions (CASAA). [Google Scholar]
- Naar-King S, Suarez M. Motivational interviewing with adolescents and young adults. New York: Guilford Press; 2011. [Google Scholar]
- Newton A, Mushquash C, Krank M, Wild T, Dyson M, Hartling L, Stewart S. When and how do brief alcohol interventions in primary care reduce alcohol use and alcohol-related consequences among adolescents? J Pediatr. 2018;197:221–232. [DOI] [PubMed] [Google Scholar]
- Otsuka America Pharmaceutical I. An overview of the ABILIFY MYCITE system. 2017; www.abilifymycite.com. Accessed July 10, 2018.
- Patel NC, DelBello MP, Keck PE, Strakowski SM. Ethnic differences in maintenance antipsychotic prescription among adolescents with bipolar disorder. J Child Adol Psychop. 2005;15:938–946. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: A review of the past 10 years. J Am Acad Child Psy. 2006;44 846–871. [DOI] [PubMed] [Google Scholar]
- Riekert K, Rand CS. Electronic monitoring of medication adherence. When is high-tech best? J Clin Psychol Med S. 2002;9:25–34. [Google Scholar]
- Schaefer MR, Kavookjian J. The impact of motivational interviewing on adherence and symptom severity in adolescents and young adults with chronic illness: A systematic review. Patient Education and Counseling. 2017;100(12):2190–2199. [DOI] [PubMed] [Google Scholar]
- Scott J, Pope M. Nonadherence with mood stabilizers: Prevalence and predictors. J Cln Psychiat. 2002;63:384–390. [DOI] [PubMed] [Google Scholar]
- Stepanova E, Findling R. Psychopharmacology of bipolar disorders in children and adolescents. Pediatr Clin N Am. 2017;64:1209–1222. [DOI] [PubMed] [Google Scholar]
- Strait G, McQuillin S, Smith B, Englund J. Using motivational interviewing with children and adolescents: a cognitive and neurodevelopmental perspective. Advances in School Mental Health Promotion. 2012;5(4):290–304. [Google Scholar]
- Taddeo D, Egedy M, Frappier J. Adherence to treatment in adolescents. Paediatric Child Health. 2008;13(1):19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Meter A, Moreira A, Youngstrom E. Meta-analysis of epidemiologic studies of pediatric bipolar disorder. J Clin Psychiat. 2011;72(9):1250–1256. [DOI] [PubMed] [Google Scholar]
- Velligan DI, Weiden P, Sajatovic M, Scott J, Carpenter D, Ross R, Docherty J. Expert consensus panel on adherence problems in persistent mental illness. J Clin Psychiat. 2009;70(supp 4):1–46. [PubMed] [Google Scholar]
- Yazdi K, Unterlass G, Kemmler G, Kralovec K, Aichorn W. Factors influencing adherence in children and adolescents treated with antipsychotics or antidepressants. J Clin Psychiat. 2008;10(2):160–161. [DOI] [PMC free article] [PubMed] [Google Scholar]


