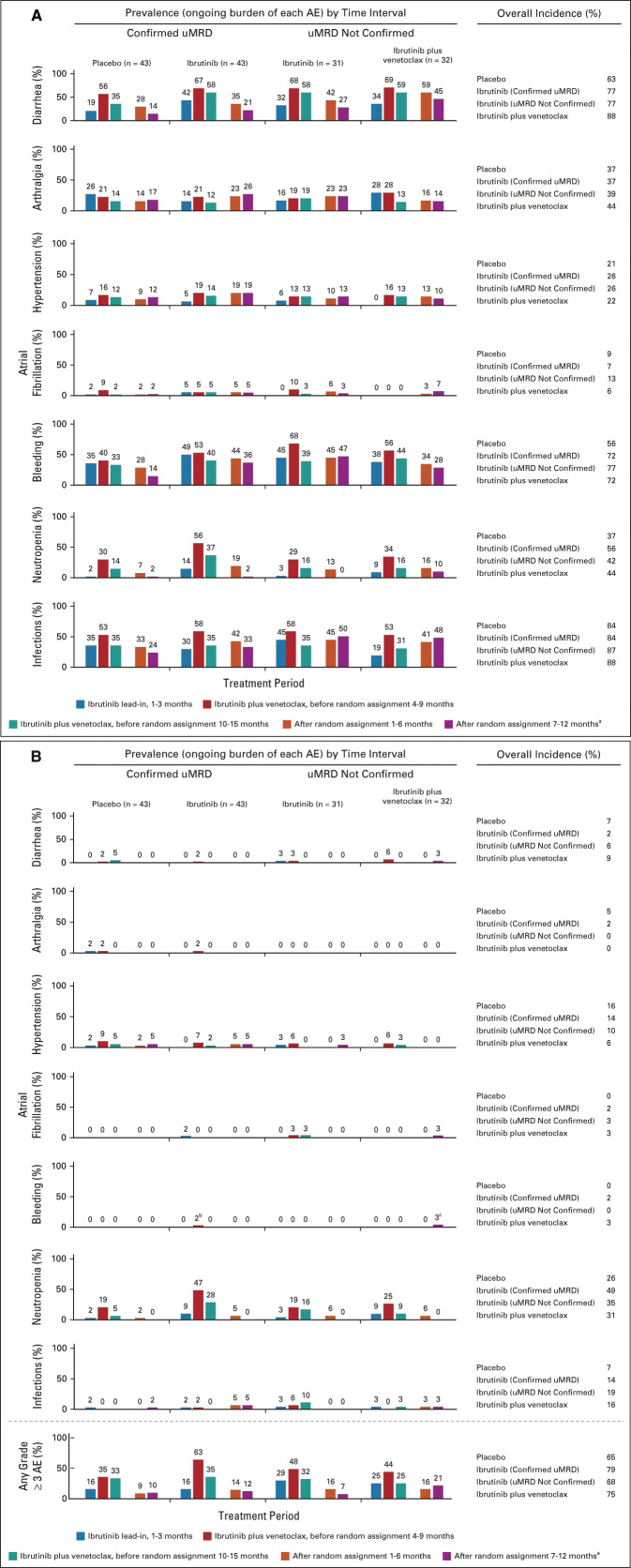
FIG 5.
Rates over time of AEs of clinical interest. (A) Prevalence of AEs of clinical interest over time by randomized treatment arm for any-grade AEs and (B) grade ≥ 3 AEs. aAt postrandomization 7-12 months, Confirmed uMRD population: placebo (n = 42), ibrutinib (n = 42); uMRD Not Confirmed population: ibrutinib (n = 30), ibrutinib plus venetoclax (n = 29). bGrade 3 menorrhagia in one patient. cGrade 3 retroperitoneal hemorrhage in one patient. AEs, adverse events; uMRD, undetectable minimal residual disease.