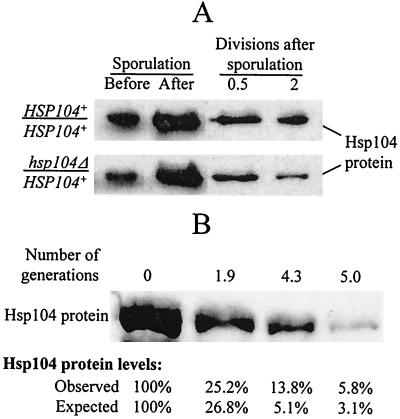
FIG. 1.
Loss of Hsp104 protein after inactivation of the HSP104 gene. (A) Fluctuations of Hsp104 levels during sporulation and germination. The diploid cultures of GT81 (HSP104+/HSP104+) and GT84 (HSP104+/hsp104Δ) were sporulated in liquid sodium acetate medium. The ascii were destroyed by lyticase treatment followed by intense vortexing, and the ascospores were shifted to the synthetic glucose medium to germinate and resume growth. Aliquots of cultures were taken at the following time points: (i) before sporulation; (ii) after 3 days of incubation in the sporulation medium, when more than 90% of cells produced ascii; and (iii) at various time points after the shift to glucose medium. Proteins were isolated, run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and reacted to Hsp104 antibody. Coomassie staining was used to verify that equal amounts of total protein were loaded in each case. Both strains contain similar amounts of Hsp104 protein at the earlier stages of germination. However, the difference between the GT81-originated culture (containing only HSP104+ cells) and the GT84-originated culture (containing a mixture of HSP104+ and hsp104Δ cells) becomes evident after two mitotic divisions following germination. (B) Loss of Hsp104 protein after inactivation of the HSP104 gene in the mitotically dividing cells occurs by dilution rather than by degradation. The hsp104Δ strains GT241-2B and GT241-3B were transformed separately with the plasmid pH28 (HIS3-GAL-HSP104). Cultures were grown in −His/Gal+Raf for 20 to 22 h to induce GAL-HSP104 and then were washed and shifted to −His/Glu, where GAL-HSP104 is repressed. The control experiment (not shown) confirmed that no detectable Hsp104 is produced by GAL-HSP104 on glucose medium. Total protein lysates were collected at various time points. Proteins were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were reacted to Hsp104-specific antibodies. Relative Hsp104 protein amounts were quantified by using densitometry as described in Materials and Methods. Observed levels are reported as the percentage of protein remaining relative to point 0 (time of shift to −His/Glu). Expected values in percentages (E) was determined from the equation E = 100/(2X) where X is the number of generations, under the assumption that no protein degradation occurs. Data confirm that a decrease in Hsp104 levels occurs due to dilution rather than degradation.