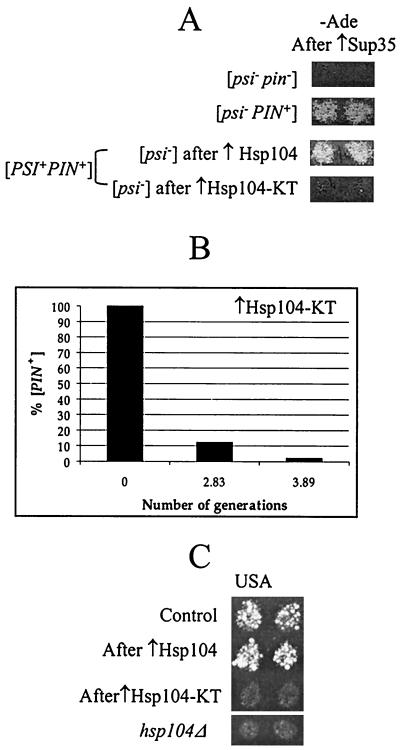
FIG. 2.
Curing of [PSI+], [PIN+], and [URE3] by ATPase-inactive mutant Hsp104 (Hsp104-KT). (A) Hsp104-KT cures both [PSI+] and [PIN+]. OT55 ([PSI+ PIN+]) containing the pLA1-SUP35 plasmid was transformed with either pYS104 (↑Hsp104) or pKT218,620 (↑Hsp104-KT), which led to a loss of [PSI+]. pYS104 or pKT218,620 was subsequently lost and the presence of [PIN+] was determined by Sup35 induction as described in Materials and Methods. Control strains GT17 ([psi− pin−]) and OT60 ([psi− PIN+]) are shown for comparison. (B) Quantitation of [PIN+] curing in the [psi− PIN+] strain OT60 transformed with the plasmid pRS316GAL-HSP104-KT. Expression of the GAL-HSP104-KT construct was induced on −Ura/Gal medium. Cells were plated onto −Ura/Glu medium after various periods of time. The presence of [PIN+] in resulting colonies was determined in the cross to the tester strain GT234/pSTR7, as described in Materials and Methods. [PIN+] curing is very rapid and nearly complete by the third generation. (C) Hsp104-KT cures [URE3]. The [URE3] strain YHE64 was transformed with the TRP1 plasmids pFL39-GAL-HSP104 (↑Hsp104), pFL39-GAL-HSP104-KT (↑Hsp104-KT), and a matching control plasmid. Cultures were induced on −Trp/Gal medium and were velveteen replica plated onto −Trp −Ura + USA/Glu medium selective for [URE3] (see Materials and Methods). In contrast to transient overproduction of wild-type Hsp104, transient overproduction of Hsp104-KT cures yeast cells of [URE3]. For comparison, the hsp104Δ derivative of YHE64 on −Ura + USA/Glu medium is shown.