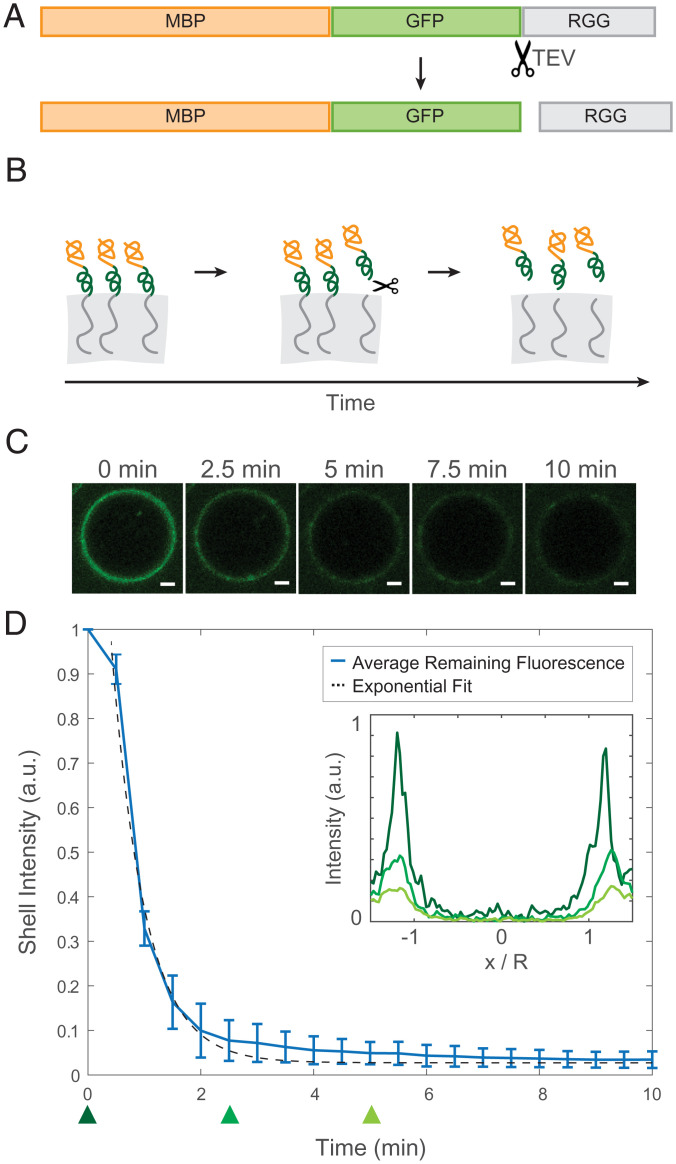
Fig. 2.
Desorption of surface-associated amphiphilic protein triggered by proteolytically cleaving the dissimilar domains. (A) Schematic depicting the location of the TEV protease cleavage site inserted into MBP-GFP-RGG between the GFP and RGG domains. (B) Schematic model of the result of adding TEV protease to the RGG-RGG and MBP-GFP-RGG system. Cleavage of the amphiphilic protein results in the release of the fluorescently tagged MBP domain into the aqueous solution and retention of the RGG domain by the condensed phase. (C) Representative droplet images at 0, 2.5, 5, 7.5, and 10 min after TEV protease addition. (Scale bars, 1 μm.) (D) Total fluorescence intensity of the shell region decreases over time with the release of MBP-GFP following cleavage of MBP-GFP-RGG by TEV protease, which was added at t = 0 (plot shows average of n = 87 droplets). (Inset) Normalized average fluorescence intensity line profiles at time 0 (dark green), 2.5 min (medium green), and 5 min (light green). DTT was added to the buffer to a final concentration of 5 mM, in accordance with recommended TEV protease protocols.