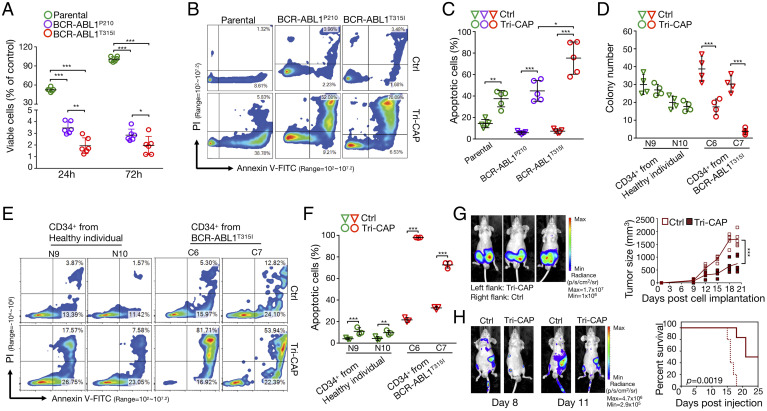
Fig. 4.
Tri-CAP induces selective apoptotic death of resistant BCR-ABL1T315I cells. (A) Viability of BCR-ABL1+ and parental cells at 24- and 72-h post–Tri-CAP treatment for 60 s (n = 6). Flow cytometry analysis of apoptosis (B) and percent apoptotic cells at 24-h post–Tri-CAP treatment in BCR-ABL1+ and parental cells (n = 5) (C). (D) Number of colonies formed 7 to 14 d following Tri-CAP treatment with primary CD34+ hematopoietic stem and progenitor cells from CML patients with T315I (C6 and C7) and cells from healthy individuals (N9 and N10); cells are described in SI Appendix, Table S6 (n = 4). Flow cytometry analysis of apoptosis (E) and percent apoptotic cells at 24-h post–Tri-CAP treatment for primary CD34+ hematopoietic stem and progenitor cells from CML patients with T315I and healthy controls (n = 3) (F). (G) Mouse tumor model of CML: Left panel shows bioluminescence images of tumors on day 20 in engrafted nude mice that were subcutaneously injected with BCR-ABL1T315I cells, with (left flank) or without (right flank) Tri-CAP treatment; graph of tumor size over time is shown at right (n = 5). (H) In the mouse survival model of CML, Tri-CAP–treated or untreated (Ctrl) BCR-ABL1T315I cells were injected into the tail veins of nude mice; left panel shows bioluminescence images of nude mice engrafted with Ctrl and Tri-CAP–treated BCR-ABL1T315I cells on days 8 and 11, and right panel shows percent survival of engrafted mice over time (n = 5). *P < 0.05, **P < 0.01, and ***P < 0.001.