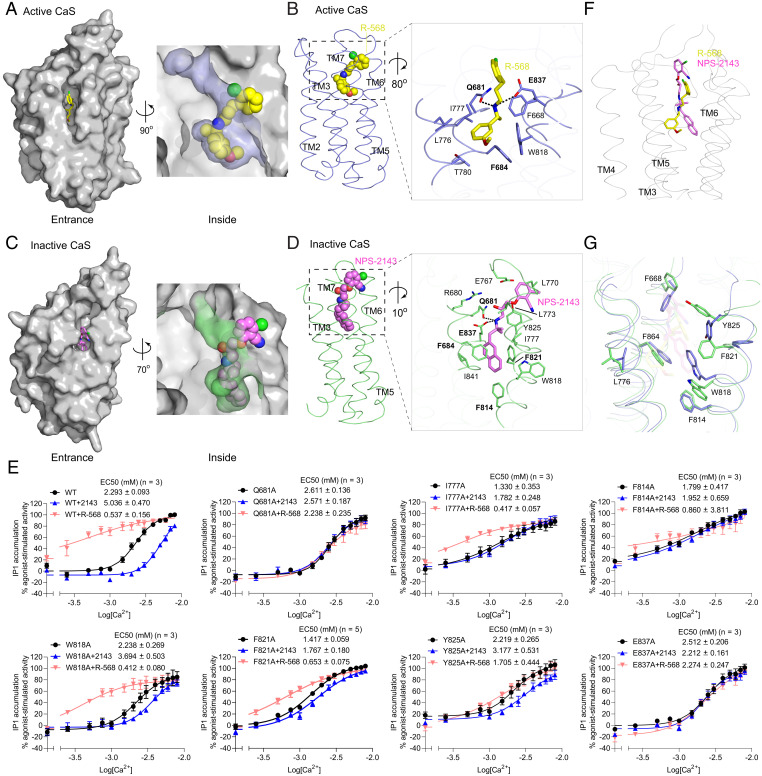
Fig. 4.
PAM- and NAM-binding sites of the CaS receptor. (A and C) Molecular surfaces of the active (A) and inactive (C) CaS TM domain, showing the binding pockets for the PAM R-568 (yellow) (A) and the NAM NPS-2143 (magenta) (C). (B and D) Binding modes of R-568 (B) and NPS-2143 (D), with the magnified views showing the specific contacts between the CaS receptor and each modulator. Hydrogen bonds are denoted by dotted lines. (E) Functional analysis of the modulator-binding sites. Dose-dependent Ca2+-stimulated IP1 accumulation was measured in cells transiently expressing either WT or mutant CaS receptor carrying one of the selected substitutions at the modulator-binding sites. The functional effect of each mutation was also assessed in the presence of 1 µM R-568 or 300 nM NPS-2143. Cell-surface expression levels of the Q681A, I777A, F814A, W818A, F821A, Y825A, and E837A mutants were 102, 89, 92, 89, 97, 96, and 53% of WT levels, respectively. Data points represent the average ± SEM of multiple experiments (n), each with triplicate measurements. (F) Comparison of R-568 and NPS-2143 binding poses. The Cα trace of the PAM-bound active CaS TM domain is shown in gray, and the superimposed R-568 (yellow) and NPS-2143 (magenta) are in stick models. The inactive CaS TM domain was overlapped onto the active form to bring their bound modulators into superposition. (G) Comparison of key residue conformations in the PAM- and NAM-binding sites of the CaS receptor. The TM domains of the active (blue) and inactive (green) CaS receptor are aligned.