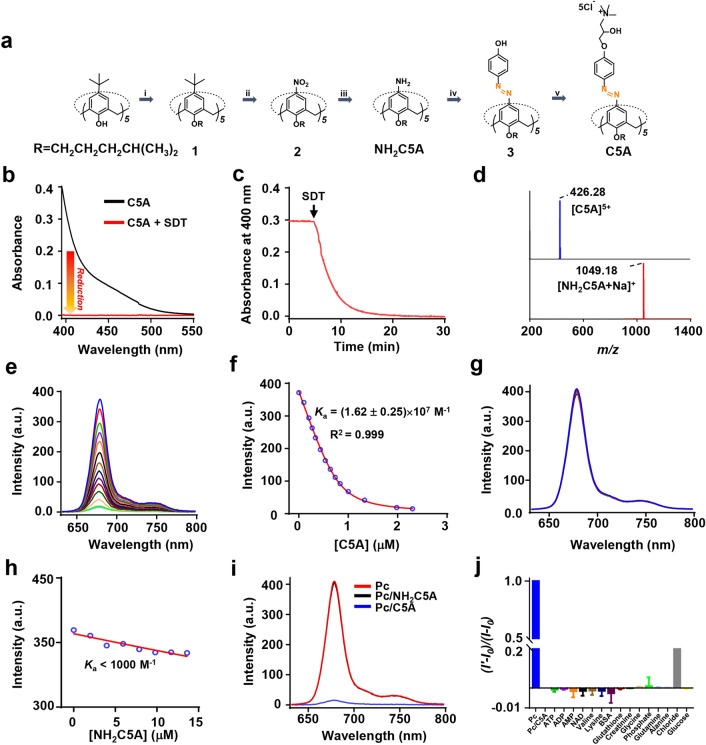
Fig. 1.
Synthesis, hypoxia response and molecular recognition of C5A. a C5A synthesis route. (i) K2CO3, RBr, CH3CN, reflux, yield 72%; (ii) AcOH, HNO3, dry CH2Cl2, 25 °C, yield 46%; (iii) SnCl2•2H2O, C2H5OH/AcOEt (1:1, v/v), reflux, yield 52%; (iv) NaNO2, HCl (aq), 0 °C; phenol, pyridine in tetrahydrofuran (THF), 25 °C, yield 64%; (v) isopropanol, glycidyltrimethylammonium chloride, reflux, yield 82%. b Absorbance of C5A (10 µM) before or after being reduced by SDT (2 mM). c C5A (10 µM) absorbance at 400 nm as a function of time following the addition of SDT (2 mM). d Mass spectra of C5A (10 µM) before (top) and after (bottom) incubation with SDT (2 mM). e Direct fluorescence titration of Pc (0.8 µM, λex = 606 nm, λem = 678 nm) with C5A (up to 2.3 µM) and f the associated titration curve fitting according to a 1:1 binding stoichiometry. g Direct fluorescence titration of Pc (0.8 µM, λex = 606 nm, λem = 678 nm) with NH2C5A (up to 13.62 µM) and h the associated titration curve fitting according to a 1:1 binding stoichiometry. The changes in fluorescence emission were too small to obtain a reasonable association constant. Therefore, the constant was estimated to be less than 1,000 M−1 according to the fitting equation [50]. i Fluorescence spectra of Pc (0.8 µM, λex = 606 nm, λem = 678 nm), Pc/NH2C5A (0.8 and 2 µM, respectively), and Pc/C5A (0.8 and 2 µM, respectively). j Fluorescence responses of Pc/C5A (2 µM each) upon addition of biologically coexisting species in blood. I is the fluorescence of Pc alone, and I0 is the fluorescence of Pc/C5A, and I’ is the fluorescence of Pc/C5A upon addition of the species. The experiments in b-j were performed at 25 °C in PBS (pH = 7.4, 10 mM)