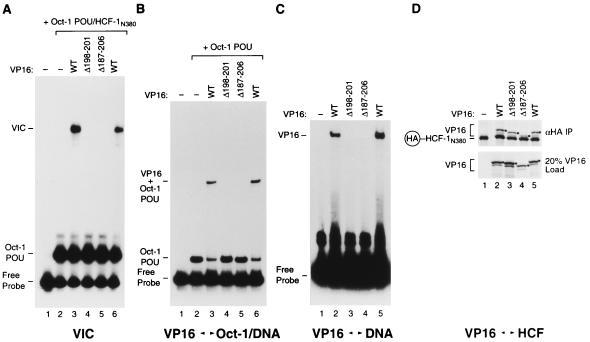
FIG. 8.
The VP16 core headrest is important for DNA binding. Wild-type (WT) and mutant (Δ198–201 and Δ187–206) VP16 molecules were tested for VP16-induced complex formation (A) and for cooperative binding to the Oct-1 POU domain–DNA complex (B), DNA alone (C), and HCF-1 (D). (A) VP16-induced complex formation was assayed as described in the legend to Fig. 5. The presence or absence of wild-type or mutant VP16 along with the Oct-1 POU domain and HCF-1N380 is indicated above each lane. (B) The ability of VP16 to interact cooperatively with the Oct-1 POU domain was assayed as described in the legend to Fig. 7A. The presence or absence of wild-type or mutant VP16 and the Oct-1 POU domain is indicated above each lane. (C) The binding of VP16 alone to DNA was assayed as described in the legend to Fig. 7B. The presence or absence of wild-type or mutant VP16 is indicated above each lane. (D) VP16 binding to HCF-1 was assayed as described in the legend to Fig. 6. The presence or absence of wild-type or mutant VP16 and HA epitope-tagged HCF-1N380 is indicated above each lane. The black dots indicate the position of the recovered VP16 proteins. The positions of the different electrophoretic species are as described in the Fig. 5 to 7 legends.