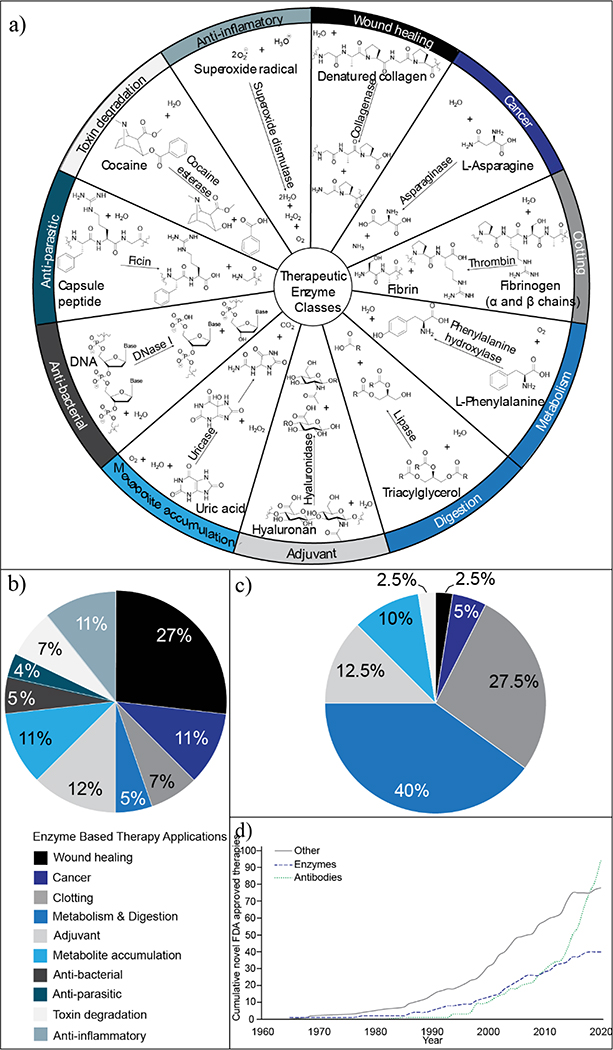
Figure 1.
Overview of enzyme-based therapies (EBTs). (a) Classes of therapeutic enzyme application and an example from each class. (b) Distribution of therapeutic enzyme applications before 1962, when the US Food and Drug Administration (FDA) approval process was established (N=32). EBT applications are listed with their corresponding colors and begin at the 12 o’clock position on the pie chart. Labeling continues in the order listed moving in the clockwise direction. For more information, see Table S2 in the supplemental information online. (c) Distribution of therapeutic enzyme applications with FDA approval in 2020 (N=39). Color and labeling scheme is consistent that in (b). For more information, see Table S3 in the supplemental information online. (d) Growth of novel biologics with FDA approval over time stratified as antibodies, enzymes, or other (such as hormones, growth factors, and cytokines). For more information, see Table S4 in the supplemental information online.