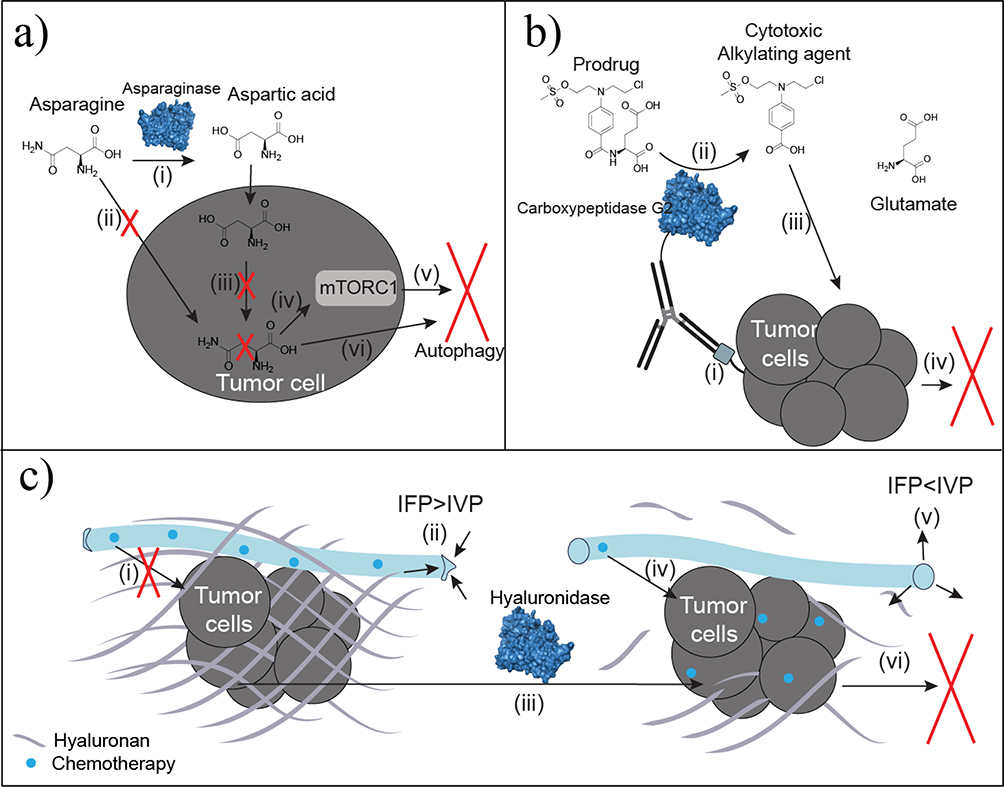
Figure 4.
Antineoplastic enzyme therapies. (a) Asparaginase converts circulating asparagine to aspartic acid (i). Asparagine depletion limits cellular uptake of asparagine (ii). Auxotrophic tumors lack asparagine synthetase needed to convert intracellular aspartic acid into asparagine (iii). Decreased intracellular asparagine regulates mammalian target of rapamycin complex 1 (mTORC1) (iv) to promote autophagy based on starvation signaling (v). Decreased asparagine also promotes autophagy by inhibiting protein synthesis because of limited precursor availability (vi). (b) Antibody-directed enzyme prodrug therapy (ADEPT) utilizes antibody specificity for irregular or overexpressed tumor antigens (i) to localize the conjugated enzyme, such as carboxypeptidase G2, at the tumor site. Carboxypeptidase G2 converts the nontoxic prodrug to the toxic benzoic acid mustard drug (ii) shown to increase potency and localization of the drug over traditional chemotherapeutic infusion. Uptake of the cytotoxic agent (iii) results in DNA alkylation and subsequent cell death (iv). (c) Thickened hyaluronan surrounding tumors is a physical barrier to chemotherapeutic diffusion (i). Increased hyaluronan also elevates interstitial fluid pressure (IFP) to be greater than intravascular pressure (IVP), which collapses the vasculature to limit diffusion and delivery (ii). Hyaluronidase degrades hyaluronan surrounding the tumor (iii). By eliminating the physical barrier, chemotherapeutics readily diffuse to improve tumor uptake (iv). Interstitial fluid pressure decreases below IVP for reperfusion and reversal of vascular collapse (v). The improved chemotherapeutic delivery and permeability improves chemotherapeutic uptake at the tumor to induce apoptosis (vi).