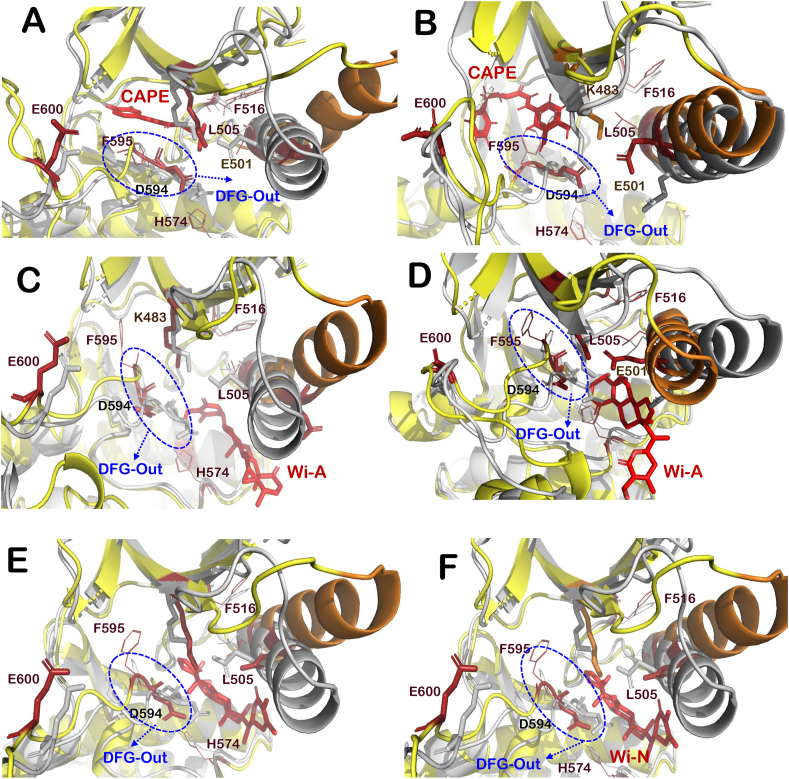
Fig. 5.
Interaction of CAPE, Wi-A and Wi-N at ATP binding site of BRAFV600Emutant protein. Superimposition of BRAFV600E protein structure (grey) with that of BRAFV600E mutant-Inhibitor complexes (yellow-red) including BRAF-CAPE complex at chain B (A) and chain C (B), BRAF-Wi-A complex at chain B (C) and chain C (D) and BRAF-Wi-N complex at chain B (E) and chain C (F). Structural elements of BRAFV600E-inhibitor complexes like activation segment (shown in firebrick color), αC-helix (orange), P loop (salmon) and R-spine residues (red lines representation). Helical turn introduced in activation segment of BRAFV600E-Wi-N complex is highlighted with cyan colored circle. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)