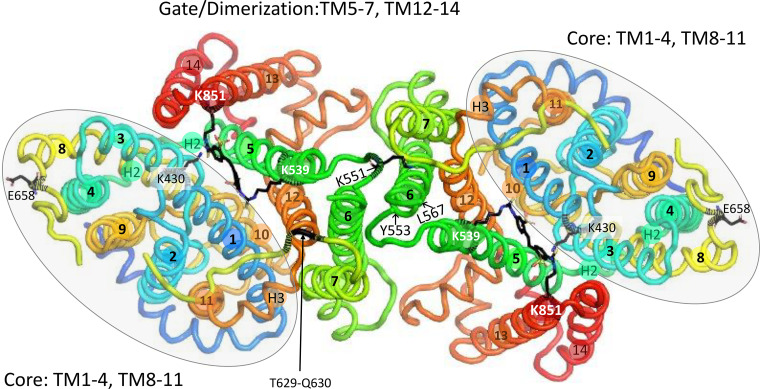
Figure 5.
Ribbon representation of the band 3 dimer crystal structure, viewed from the extracellular side of the membrane to illustrate the relationships among dimer interface, gate, core, and stilbenedisulfonate site. The core domains are in the shaded ovals. Covalently bound 4,4′-diisothiocyanatodihydrostilbene-2,2′-disulfonate (H2DIDS) between core and gate is shown in black sticks for both subunits. One of the connections between core and gate domains is cytoplasmic surface helix H2, which is largely obscured by TM8 in this view; the two ends of H3 are labeled. The second connection between core and gate consists of extracellular helix H3 and the extracellular TM7-TM8 loop. Papain cleavage of the TM7-TM8 loop between T629 and Q630 (shown for the left-hand subunit) appears to stabilize the inward-facing conformation (see text).