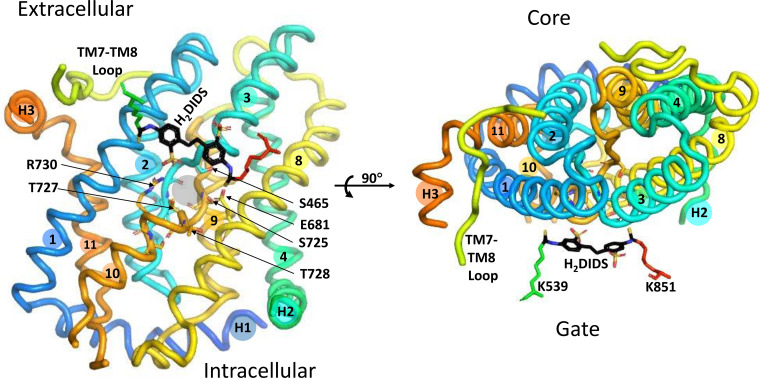
Figure 6.
Left: core domain, viewed from the gate/dimerization domain, showing the relationship between the probable substrate binding pocket (gray circle between the helical portions of TM3 and TM10) and covalently bound 4,4′-diisothiocyanatodihydrostilbene-2,2′-disulfonate (H2DIDS) (black sticks). The side chains of R730 and E681 are on either side of the substrate pocket. One of the H2DIDS sulfonate groups is near the side chain of R730. The positions of other polar side chains (S465, S725, T727, and T728) are indicated. The 3 links between core and gate domains (portion of TM7-TM8 loop and surface helices H2 and H3) are viewed end-on. Right: same structure, rotated to show the view from the extracellular medium. The only gate domain amino acid residues shown are those covalently bound to H2DIDS. The helical portion of TM10 is behind TM1 in this view. H2DIDS is between core and gate, near but not in the substrate binding pocket.