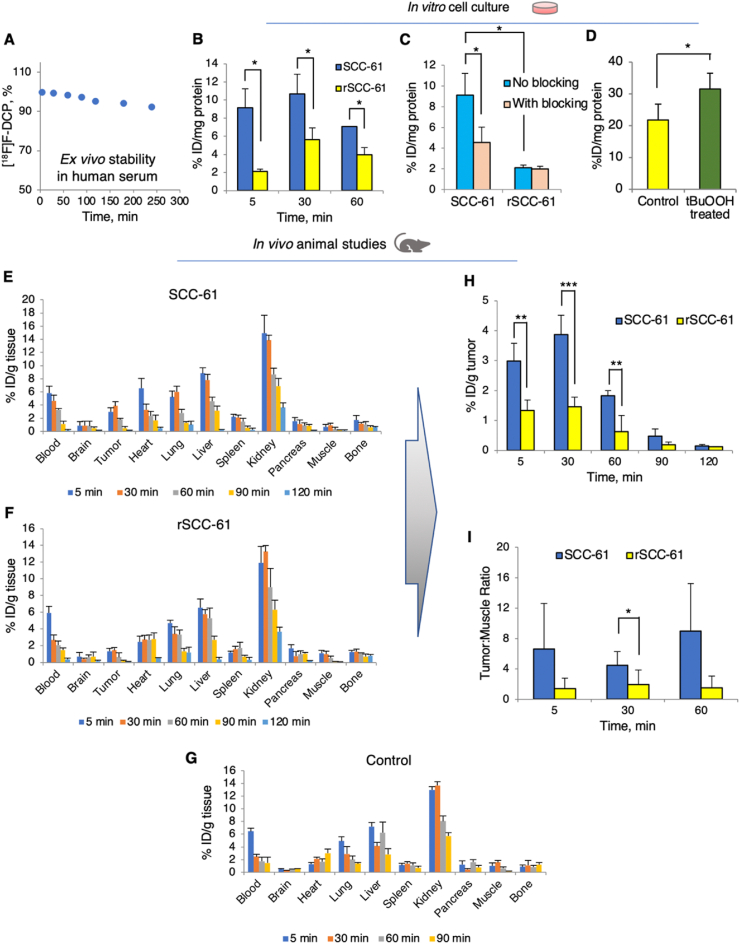
Fig. 2.
Primary characterization of [18F]F-DCP compatibility with in vivo applications. (A) Serum stability assay for [18F]F-DCP performed by incubating the radiotracer with human serum and quantifying by HPLC analysis over a 4 h time course (n = 2). (B) In vitro cell accumulation of [18F]F-DCP in SCC-61 and rSCC-61 cells after 5 min, 30 min and 60 min of radiotracer exposure (n = 3). (C) In vitro assessment of binding specificity using blocking with non-radioactive F-DCP analog in SCC-61 and rSCC-61 cells. Blocking of sulfenylated proteins was demonstrated by exposing the cells to 50x excess F-DCP, 15 min prior to adding the [18F]F-DCP radiotracer for 5 min, 30 min, and 60 min (n = 3). (D) Control experiments showing increased cellular [18F]F-DCP with oxidant treatment (tBuOOH: tert-butyl hydroperoxide; 300 μM, 15 min followed by radiotracer for 30 min) (n = 3). (E)–(I) Biodistribution of [18F]F-DCP in matched xenograft animal models of radiation response: radiation sensitive SCC-61 (E), radiation resistant rSCC-61 (F), and control non-tumor carrying animals (G) (n = 4 for each group). (H) Analysis of biodistribution data shows accumulation of [18F]F-DCP in SCC-61 tumors 2–3 fold more than in rSCC-61 tumors, a ratio stable up to 90 min post injection. (I) Tumor to muscle ratios extracted from the biodistribution data show [18F]F-DCP accumulation in SCC-61 tumors compared to rSCC-61 tumors. The data in (B)–(H) were expressed as % injected dose (ID)/mg of protein present in each well with p values ≤ 0.05 considered statistically significant. Statistical analysis (t-test) was performed using Microsoft Excel and all results are displayed as mean ± standard deviation. Asterisks indicate statistically significant changes [α = 0.05, p values of 0.01–0.05 (*), 0.001–0.01 (**), or <0.001 (***)].