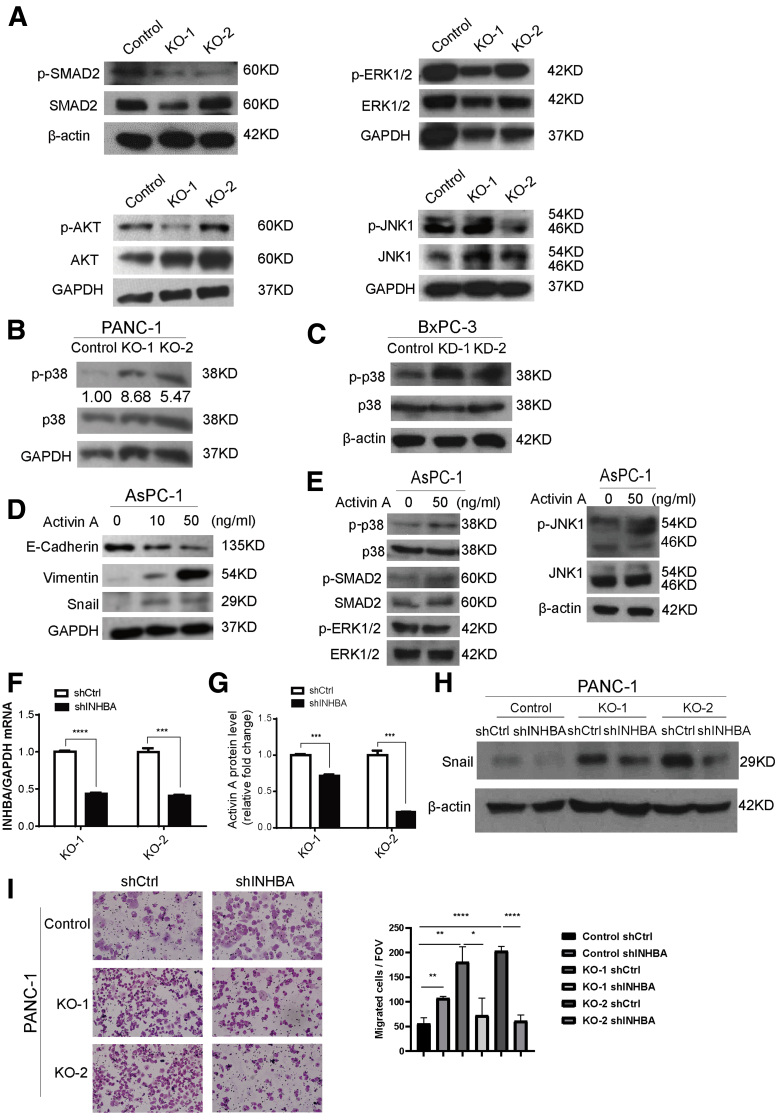
Figure 7.
Loss of KDM6A activates a noncanonical p38 MAPK-dependent activin A pathway. (A) Western blot of p-SMAD2, SMAD2, p-ERK1/2, ERK1/2, p-AKT, AKT, p-JNK1, and JNK1 in control and KDM6A-KO PANC-1 cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. (B) Western blot of p-p38 and p38 in KDM6A-KO and control PANC-1 cells and (C) KDM6A short hairpin (sh) RNA knockdown or control BxPC-3 cells. GAPDH and β-actin were used as loading controls, respectively. (D) Western blot of E-cadherin, Vimentin, and Snail in AsPC-1 cells treated with vehicle control, or 10 or 50 ng/mL of activin A for 6 hours. GAPDH was used as a loading control. (E) Western blot of p-p38, p38, p-SMAD2, SMAD2, p-ERK1/2, ERK1/2, p-JNK1, and JNK1 in AsPC-1 cells treated with vehicle control or 50 ng/mL of activin A for 6 hours. β-actin was used as a loading control. (F) Real-time reverse-transcription polymerase chain reaction of INHBA in KDM6A-KO PANC-1 cells transfected with control or INHBA shRNA. GAPDH was used as a reference gene. (G) Relative activin A protein levels in condition media from cultured KDM6A-KO PANC-1 cells transfected with either control or INHBA shRNA measured by enzyme-linked immunosorbent assay. (H) Western blot of Snail in the control and 2 KDM6A-KO PANC-1 cell clones transfected with either control or INHBA shRNA. β-actin was used as loading control. (I) Representative images and quantifications of migrated control and KDM6A-KO PANC-1 cells transfected with either control or INHBA shRNA. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001, and ∗∗∗∗P < .0001, unpaired t test. Ctrl, control; FOV, field of view; mRNA, messenger RNA.