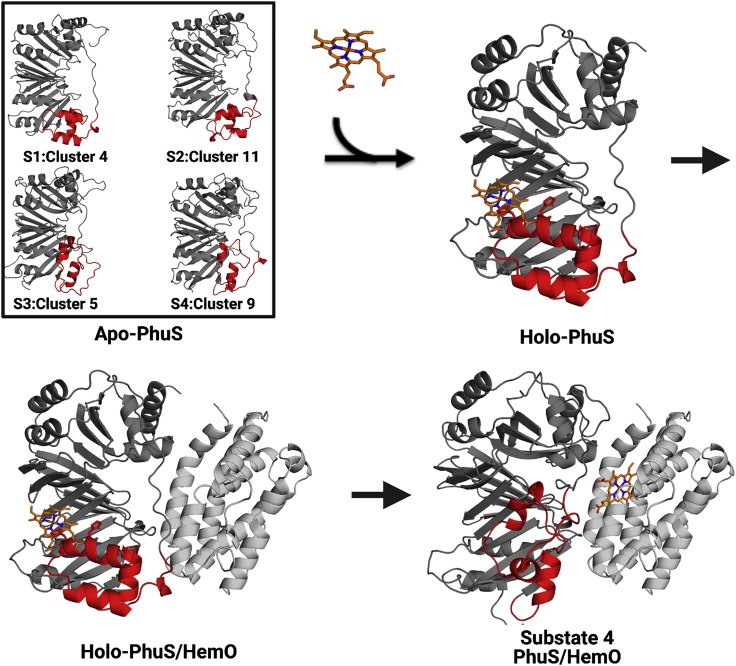
Figure 6.
The proposed mechanism of heme binding to PhuS and subsequent transfer to HemO. Apo-PhuS exists in multiple conformational substates, represented here by the four major substrates (top left) extracted by ST and HDXer. Heme binding is accompanied by a conformational rearrangement to coordinate the heme on the proximal side as in the crystallographic form (top right). Heme binding drives the formation of the holo-PhuS/HemO complex, modeled by cross-linking and mass spectrometry and docking studies (22) (bottom left). Transfer of heme to HemO may occur through a conformation in which the α6,7,8 helices reorient toward HemO; this putative conformation might consist of one similar to the representative structure from substate 4, shown aligned with PhuS within the context of the PhuS-HemO complex (bottom right). The α6,7,8 helices are colored red.