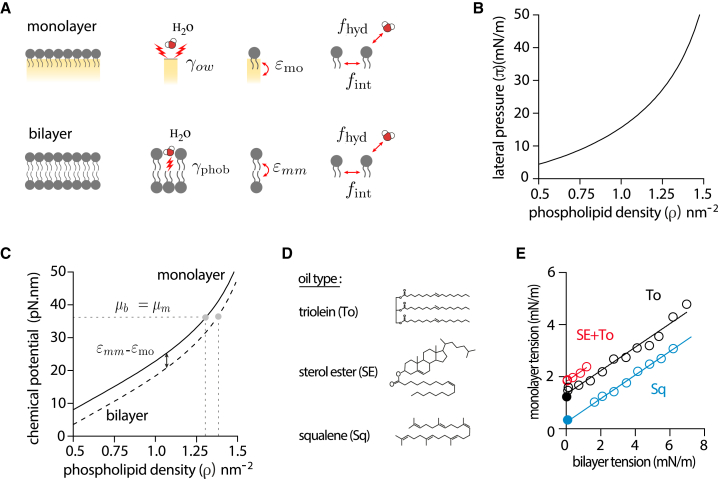
Figure 3.
Microscopic description of the contiguous droplet-bilayer system. (A) Microscopic contributions to the free energy density of a monolayer and of a bilayer. For the monolayer: the surface tension of the bare water/oil interface (), the interaction of the PL tails with the oil phase , the lateral interaction between PLs (), and the interaction of the PL headgroups with water (). For the bilayer: the unfavorable interactions of the PL tails with water (), the trans interaction between PL tails () of the apposed leaflet, the lateral interaction between PLs (), and the interaction of the PL headgroups with water (). (B) Lateral pressure as a function of PL density at the triolein-water interface deduced from a compression isotherm obtained from a pending droplet experiment (see Fig. S2A and B). (C) Chemical potential of a bilayer and a monolayer as a function of PL density. The two chemical potentials vary identically but are shifted by the amount . At equilibrium, a monolayer in contact with a bilayer has the same chemical potential () and therefore has different PL densities, whose values are directly affected by the gap. (D) Molecular structure of the different oils used to probe the effect of oil composition on monolayer-bilayer surface tension diagram. (E) Monolayer-bilayer surface tension diagram of DOPC DEVs made with different oil composition: sterol ester-triolein (1:3) (red circles), triolein (black circles), and squalene (blue circles). DEVs were selected with a droplet symmetrically positioned with respect to the bilayer. Black dot represents the average monolayer tension of osmotically deflated triolein DEVs (bilayer tension <0.03mN/m; n = 17). Blue dot represents the average monolayer tension of osmotically deflated squalene DEVs (bilayer tension <0.09mN/m; n = 9). See Fig. S2C for monolayer-bilayer tension diagram of squalene and sterol ester-triolein (1:3) DEVs with asymmetric positions of the droplet.