Figure 5.
The anticipated outcomes of the protocol
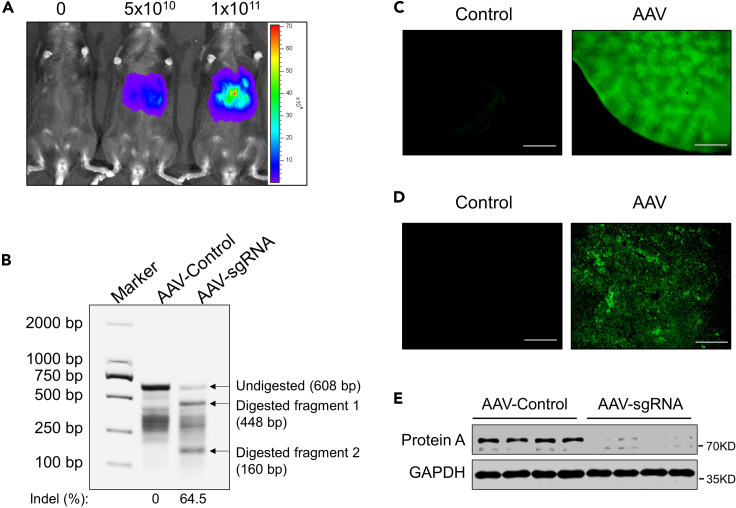
(A) In vivo bioluminescence imaging of mice one week after administration with 0, 5 × 1010, 1 × 1011 AAV-Cre-sgRNA AAVs.
(B) Gel image of liver DNA T7E1 assay after 3 weeks of infection with AAVs that does not carry (AAV-Control) and carries sgRNA (AAV-sgRNA).
(C) The images of GFP expression in fresh liver tissues from mice upon two weeks’ infection with 0 or 1 × 1011 AAV-Cre-sgRNA AAVs. Scale bars represent 1 mm. (D) The images of GFP expression in frozen liver sections from mice upon two weeks’ infection with 0 or 1 × 1011 AAV-Cre-sgRNA AAVs. Scale bars represent 250 μm.
(E) Western analysis of protein A expression in liver tissues from animals after 3 weeks’ infection with AAVs not carrying (AAV-Control) or carrying sgRNA (AAV-sgRNA).