Abstract
Syphilis rates have continued to rise in the United States. Florida and Louisiana consistently report high numbers of cases. We evaluated rates of reinfection to see if frequent rescreening might lead to earlier treatment and prevent infections. All syphilis records of all stages for males and females aged 15–70 years from the Florida and Louisiana Departments of Health surveillance databases 2000–2018 were evaluated. The first episode of syphilis during this period was considered the initial diagnosis for each person. Demographics of cases and repeaters (individuals reported with two or more cases of syphilis) were examined. Percentages of syphilis cases from repeaters by year were calculated as were percentages from HIV+ males. During 2000–2018, 124,827 syphilis cases were reported from 107,405 individuals: 73,811 (68.7%) males; 33,594 (31.3%) females. There were 12,545 individuals (repeaters) with two or more syphilis diagnoses (n = 17,422 cases; range, 2–10). From 2010 to 2018, repeaters accounted for steadily increasing percentage of all syphilis reported: 2010 (11%), 2013 (16%), 2015 (20%), and 2018 (26%). Among HIV+ male cases the percentage from repeaters also increased: 2010 (28%), 2013 (35%), 2015 (42%), and 2018 (50%). In 2018, 19% of all cases (n = 2,455) were from HIV+ males who had a previous syphilis diagnosis. Among HIV+ males diagnosed with syphilis in 2015, 34% had a repeat syphilis diagnosis within 3 years. Most syphilis diagnosed in Florida and Louisiana was among persons infected for the first time. However, some subgroups could possibly benefit from more frequent screening. Males living with HIV who had a prior syphilis diagnosis were at very high risk of repeat infection.
Keywords: syphilis, reinfection, screening, men
Introduction
Three previous syphilis epidemics in the United States (1975, 1982, and 1990) peaked within 5–6 years, but the current epidemic is still increasing after 20 years.1 Rates have risen steadily, after reaching its nadir of 2.1 per 100,000 population of infectious syphilis in 2000, which were the lowest recorded rates since syphilis surveillance started in 1941.2–4 The highest rates in 2000 were in the South and accounted for 62% of cases. Then rates of primary and secondary syphilis started increasing among men who have sex with men (MSM) many who were infected with HIV.2–7 This increase followed the release and subsequent uptake of highly effective antiretrovirals to treat HIV.7,8
In 2016, the United States Preventive Services Task Force (USPSTF) issued guidance on screening for syphilis.9 The Task Force rendered a Grade A (its highest designation) recommendation for screening for syphilis in nonpregnant adults and adolescents. Specifically, the USPSTF recommends screening for syphilis in persons who are at increased risk for infection. The Grade A designation indicates: “there is high certainty that the net benefit is substantial,” the USPSTF recommends the service, and in practice it should be offered or provided. During discussions of the evidence for its recommendation MSM living with HIV were determined to be the highest-risk group. The possibility of screening every 3 months was mentioned but it was not included in the formal recommendation. The recommendation suggested that clinicians should make the decision about screening based on community prevalence.
Several studies examining the influence of syphilis reinfections were published in the first decade of this epidemic finding that reinfections had a moderate but rising effect on syphilis rates.4,10 These studies were conducted in an earlier phase of the epidemic and covered short time periods of 3–8 years. We examined all syphilis records from two states that consistently report high syphilis morbidity, Florida and Louisiana, to explore the influence of reinfections on rates for the past 18 years.
Methods
Syphilis is a notifiable condition in all US states.11 Positive syphilis tests are reported by laboratories and syphilis diagnoses are reported by clinicians. The individual’s name, test results, and demographic information including the ordering provider are required for reporting. These reports are entered into statewide surveillance registries. Surveillance personnel compare reported test results with previous test results and treatment information to see if the test suggests a new infection. Personnel also cross match their HIV/AIDS registries with their sexually transmitted disease surveillance registries to determine if individuals are co-infected. After the record search, if the test result is suspected to be a new case, surveillance personnel contact the provider to gather information on signs and symptoms, diagnosis, treatment, demographics, and how to locate the potential case. Then disease investigation specialists contact the individual for interview. New information on diagnosis, stage, and treatment are recorded in the registry.
We conducted a retrospective cohort study of all syphilis records, all stages (early, nonprimary nonsecondary, and unknown duration or late) for males and females aged 15–70 years from January 1, 2000 to December 31, 2018. Demographics of cases and repeaters (individuals reported with two or more cases of syphilis), including age, race/ethnicity, gender, and HIV status were examined. We did not have access to a sexual orientation variable, which was incomplete in the early years of the study. Time to second infection and number of subsequent infections were assessed for repeaters. We calculated the percentages of syphilis cases that were from repeaters each year as well as percentages of cases from people living with HIV (PLWH). We also assessed the likelihood of reinfection within 3 years for persons with an initial diagnosis of syphilis in 2015.
All data management and statistical analyses were conducted using Statistical Analysis Software (version 9.4 for Windows; SAS Institute Inc., Cary, NC).12 The figures were created using R (version 4.0.4) in R Studio (version 1.4.1103) using the “ggplot2” package.13–15
Centers for Disease Control and Prevention (CDC) did not have access to personal identifiers. This was an evaluation of routinely collected public health program data and, therefore, received a nonresearch determination by the CDC. This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy: 45 C.F.R. part 46 102(1)(2), 21 C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
Results
From 2000 to 2018, 124,827 syphilis cases were reported to the states of Florida and Louisiana from men and women between the ages of 15 and 70. These cases were diagnosed from 107,405 individuals: 73,811 (68.7%) males and 33,594 (31.3%) females (Table 1). Florida contributed 90,279 (72.3%) of the cases; from 75,706 (70.5%) individuals. There were 94,860 (88.3%) persons with a single episode of syphilis reported and 12,545 (11.7%) from individuals with two or more syphilis diagnoses reported (repeaters). Diagnoses among persons who had a previous diagnosis accounted for 17,422 (14%) of all cases (this case count does not include their initial diagnosis). Including their initial diagnosis, repeaters accounted for 29,967 (24%) of all cases reported. Individuals had up to 10 total infections. Mean time to reported second infection was 3.6 years (median = 2.6 years). Over time, repeaters accounted for a steadily increasing percentage of all syphilis reported: 11% in 2011, 16% in 2013, 20% in 2015, and 26% by 2018 (Fig. 1).
Table 1.
Demographics of All Persons with Single Episodes and Repeat Episodes of Syphilis
| All syphilis | Single episode | Repeaters | Repeat riska % | ||||
|---|---|---|---|---|---|---|---|
| N = 107,405 | %b | N = 94,860 | %b | N = 12,545 | %b | ||
| Site | |||||||
| Florida | 75,706 | 70.5 | 65,531 | 69.1 | 10,175 | 81.1 | 13.4 |
| Louisiana | 31,699 | 29.5 | 29,329 | 30.9 | 2370 | 18.9 | 7.5 |
| Gender | |||||||
| Male | 73,811 | 68.7 | 62,810 | 66.2 | 11,001 | 87.7 | 14.9 |
| Female | 33,594 | 31.3 | 32,050 | 33.8 | 1544 | 12.3 | 4.6 |
| Race/ethnicity | |||||||
| White | 23,479 | 21.9 | 20,139 | 21.2 | 3340 | 26.6 | 14.2 |
| Black | 49,519 | 46.1 | 45,088 | 47.5 | 4431 | 35.3 | 8.9 |
| Hispanic | 19,929 | 18.6 | 16,750 | 17.7 | 3179 | 25.4 | 16.0 |
| Asian, Indian, PI | 733 | 0.7 | 645 | 0.7 | 85 | 0.7 | 11.6 |
| Other/unknown | 13,745 | 12.8 | 12,235 | 12.9 | 1510 | 12.4 | 11.0 |
| Age, yearsc | |||||||
| 15–19 | 7013 | 6.5 | 6161 | 6.5 | 852 | 6.8 | 12.1 |
| 20–29 | 35,863 | 33.4 | 31,527 | 33.2 | 4336 | 34.6 | 12.1 |
| 30–39 | 27,794 | 25.9 | 24,307 | 25.6 | 3487 | 27.8 | 12.5 |
| 40–49 | 21,635 | 20.1 | 18,908 | 19.9 | 2727 | 21.7 | 12.6 |
| 50–70 | 15,100 | 14.1 | 13,597 | 14.7 | 1143 | 9.1 | 7.6 |
| HIV status | |||||||
| Negative | 82,507 | 76.8 | 76,877 | 81.0 | 5630 | 44.9 | 6.8 |
| Positive | 24,898 | 23.2 | 17,983 | 19.0 | 6915 | 55.1 | 27.8 |
Repeat risk is a row percent calculated: Repeaters#/All Syphilis# × 100. All other percents are for columns.
All percent columns may not sum to 100 due to rounding.
Age for “All Syphilis” and “Single Episode” is age at first diagnosis on or after January 1, 2000. Age for “Repeaters” is age at time of the second diagnosis.
PI, Pacific Islander.
FIG. 1.
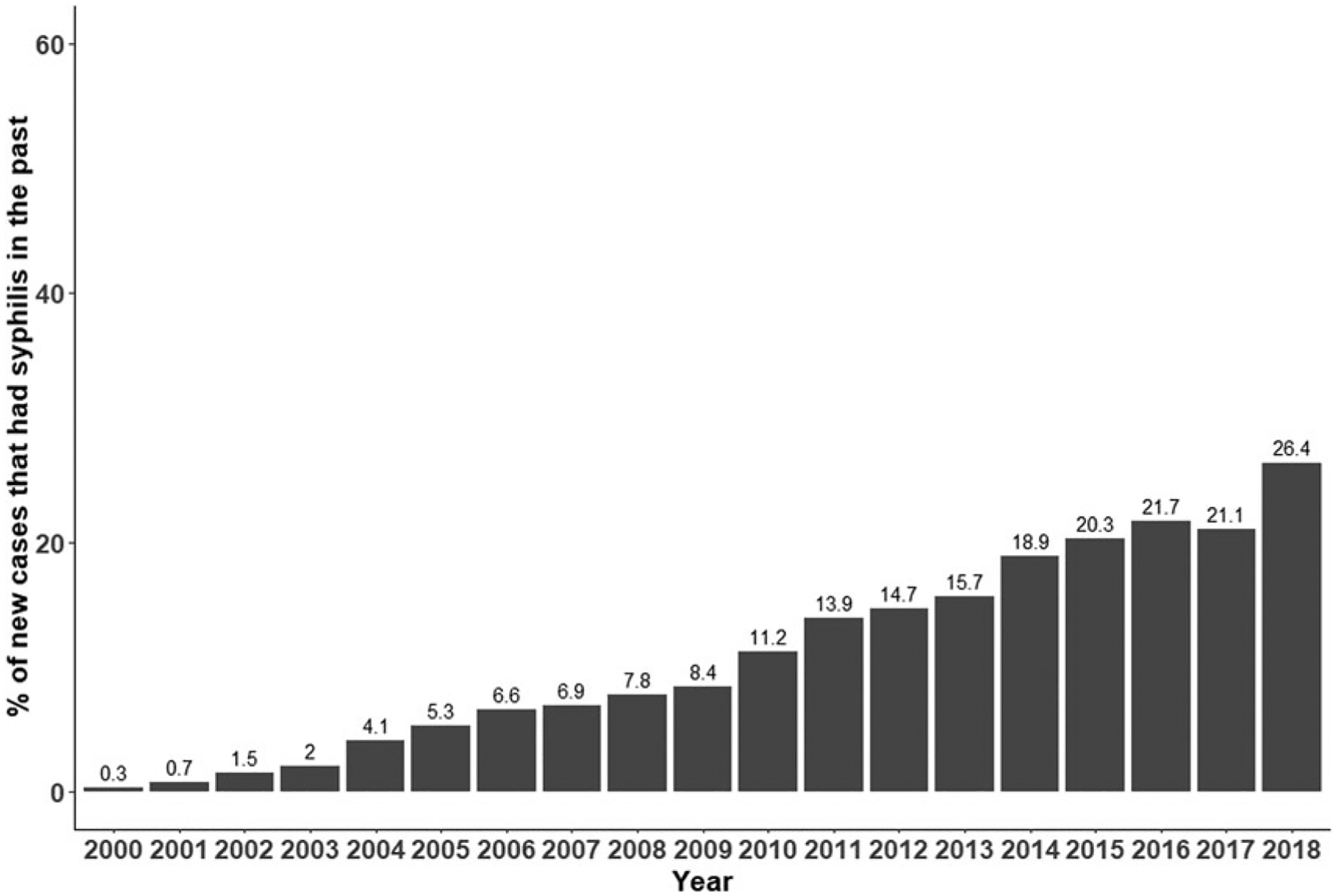
Among all new cases of syphilis, the percent that had syphilis in the past.
Repeat infection was more common among men with syphilis (14.9%) compared with women (4.6%). Repeaters accounted for a higher percentage of all infections: for Hispanics (16.0%) and non-Hispanic Whites (Whites) (14.2%), than non-Hispanic Blacks (Blacks) (8.9%). The likelihood of repeat infection was remarkably consistent across all age categories until it decreased for persons >50 years. Repeat syphilis was much more likely among HIV-positive persons with syphilis (27.8%) compared with HIV-negative persons with syphilis (6.8%).
There were 1,544 women with repeat infections that accounted for 12.3% of all repeaters and 1685 (9.7%) of all repeat episodes reported (range, 2–5 cases). These repeat infections among women represent 1.3% of all cases. Mean time to second infection was 3.5 years (median = 2.3 years). From 2010 to 2018, female repeaters accounted for a slowly increasing percentage of all syphilis reported: 2010 (5.1%), 2013 (5.9%), 2016 (6.8%), and reached (10.3%) in 2018. For syphilis among females living with HIV, cases from repeaters also increased: 2010 (13%), 2014 (16%), 2016 (21%), and 2018 (25%) (Data not shown).
The 11,001 male repeaters accounted for 87.7% of all repeaters and 15,737 (90.3%) of all repeat episodes (range of 2–10 infections). Mean time to second infection for these men was 3.7 years (median = 2.6 years). Male repeaters accounted for an increasing percentage of syphilis cases reported over time: 2010 (14%), 2013 (19%), 2015 (24%), and in 2018 (30%). For syphilis among males living with HIV, the percentage of cases from repeaters also increased: 2010 (28%), 2013 (35%), 2015 (42%), and 2018 (50%) (Fig. 2). In 2018, 19% of all cases were from 2,455 HIV+ males that had a previous syphilis diagnosis.
FIG. 2.
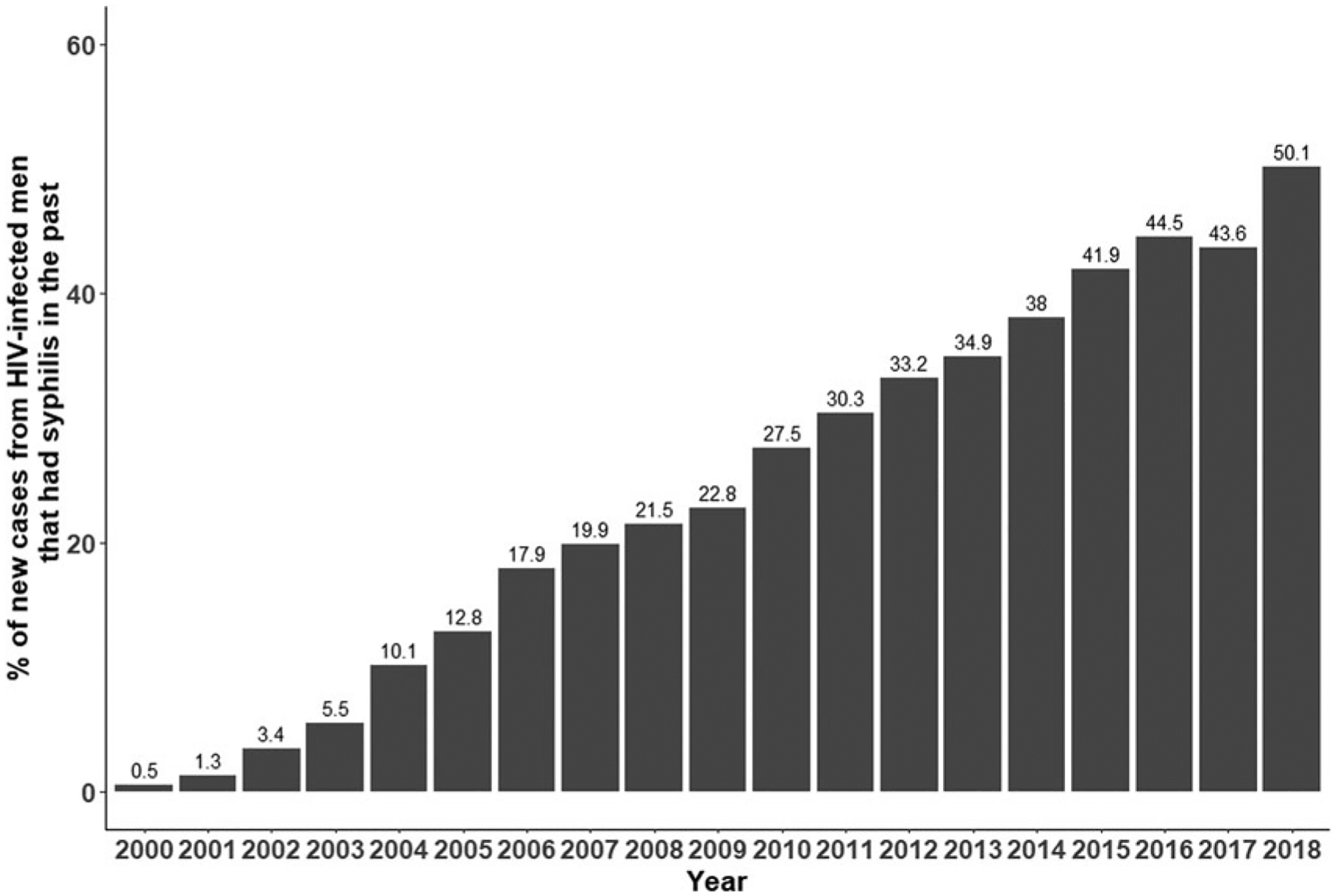
For new cases of syphilis among HIV-infected men, the percent that had syphilis in the past.
We conducted a subset analysis looking at individuals infected in 2015 to see how many became reinfected by 2018. Among the individuals diagnosed with syphilis in 2015, 18% became reinfected within 3 years; for males, 21% became re-infected; for HIV+ males, 34% had a repeat syphilis diagnosis within 3 years; for women, 5% became reinfected by 2018 and 8% of HIV+ women were reinfected within the 3 years.
Discussion
Syphilis reinfections are not new or recent phenomena. But our study indicates over an extended period of years in this latest epidemic that the proportion of reinfections have dramatically increased. We found 26% of all infections in 2018 were reinfections. And for HIV+ men, 50% of all infections in 2018 were reinfections. This suggests that frequent rescreening of persons after the diagnosis of syphilis would lead to earlier diagnosis and might help decrease transmission. When we tested this with persons diagnosed in 2015, we found 18% were reinfected within 3 years, and for HIV+ men 34% were reinfected within 3 years. Women’s risk of repeat syphilis was lower than males (5% within 3 years of 2015). In a previous study of syphilis reinfections in Florida between 2000 and 2008, 2.5% of all syphilis diagnoses were from repeaters.4 Other studies of repeaters have reported varying time periods covering different years making direct comparisons difficult. They reported reinfection rates of between 2.5% and 17.6%.4,10,16–19
In 2000, the current syphilis outbreak coincided with the uptake of antiretroviral therapy for the treatment of HIV.7 Researchers have also investigated the influence of pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) in MSM for the prevention of HIV and reported on the subsequent increase in sexually transmitted infections (STIs).20–24 PrEP and PEP arrived in a time of electronic applications (APPS) to meet sexual partners, possible change in sexual behaviors of less condom use and increase in condomless anal intercourse (CAI).23,24 One comprehensive study of CAI, partnership type and shared biomedical disclosure (PrEP use), found that as both partners were using PrEP CAI increased compared with one partner using PrEP or no partners disclosing PrEP use.23 This association was not found in partnerships of non-PrEP using MSM. The authors concluded that as “PrEP matching” increases in MSM the practice could contribute to disproportionate STI risk.23
A study among PLWH in military personnel between 2014 and 2017 found that 18.9% of participants who completed a year of follow-up acquired an STI.25 More than a quarter (27.2%) of the infections reported were syphilis. A study of PLWH reported from a Kaiser Mid-Atlantic, a large nonprofit health maintenance organization (HMO), called for more quality of care measures (QMs) beyond viral suppression to improve process metrics with ultimate goal of improving outcome metrics.26 The military and HMO studies of PLWH/PWH illustrate even with people knowledgeable of STI risk and access to quality health care is not sufficient. Broader quality of care measures (QMs) and a holistic health approach may eventually lead to better outcomes.26 Currently, the USPSTF recommends screening people at high risk, we have quantified groups and subgroups at extremely high risk.9 As syphilis continues to rise, more frequent screening of men with a past history of syphilis and in particular more screening of HIV+ men with a past history of syphilis may be warranted to attempt to better control this latest epidemic that has been with us approaching 20 years. Further research to quantify the optimal time between screenings should be pursued for the most at risk groups.
SYPHILIS REINFECTION
Other researchers have developed innovative approaches to combat the increasing syphilis rates and other STIs in MSM.27–30 They have assessed the feasibility and conducted pilot trials of chemoprophylaxis to prevent syphilis and other STIs. Their focus has been on core groups and the ongoing use of doxycycline prophylaxis to prevent incidence and the associated transmission. Our findings support concentrating these efforts on HIV-infected men who have had syphilis in the past.
Our study has some limitations. Some infections may remain undiagnosed. We included cases diagnosed in Florida and Louisiana reported to the State Departments of Health from 2000 to 2018, so the first episode of syphilis during this period was considered the initial diagnosis although some likely had a prior diagnosis before 2000. Likewise, persons diagnosed late in the study period (e.g., 2018) would not have had the opportunity to repeat. There may have been in and out migration of people from the states influencing whether all syphilis cases were diagnosed and reported. We did not have complete sexual orientation data. PLWH may get screened more often for syphilis as they have more opportunities to be screened due to monitoring of their HIV treatment.
This study has strengths as well. To our knowledge this is the longest study of syphilis reinfection (18 years). The study includes data from the beginning of the ongoing long epidemic. We were able to examine syphilis records from two states, which strengthen that the results were not an anomaly seen in only one state.
There has been a steady and dramatic increase in the proportion of new syphilis cases that are in persons who have had syphilis in the past. In 2018, 26% of all infections and 50% of infections among HIV+ men are contributed by persons with past syphilis. Frequent screening of this small number of individuals could identify infections early and might reduce onward transmission.
Funding Information
No funding was received for this study.
Footnotes
Author Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Centers for Disease Control and Prevention. The National Plan to eliminate syphilis from the United States. Atlanta, GA: US Department of Health and Human Services, CDC, National Center of HIV, STD, and TB Prevention, 1999. Available at: http://www.cdc.gov/stopsyphilis/plan.pdf (Last accessed February 1, 2021). [Google Scholar]
- 2.Centers for Disease Control and Prevention. Primary and secondary syphilis: United States, 2000–2001. MMWR Morb Mortal Wkly Rep 2002;51:971–973. [PubMed] [Google Scholar]
- 3.Brewer TH, Peterman TA, Newman DR, Schmitt K. Re-infections during the Florida syphilis epidemic, 2000–2008. Sex Transm Dis 2011;38:12–17. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2018. Atlanta, GA: US Department of Health and Human Services, 2019. Available at: https://www.cdc.gov/std/stats/archive.htm (Last accessed February 5, 2021). [Google Scholar]
- 5.Klausner JD, Kent CK, Wong W, et al. The public health response to epidemic syphilis, San Francisco, 1999–2004. Sex Transm Dis 2005;32(10 Suppl.):s11–s18. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt K, Bulecza S, George D, et al. Florida’s multi-faceted response for increases in syphilis among MSM: The Miami-Ft. Lauderdale Initiative. Sex Transm Dis 2005; 32(10 Suppl.):S19–S23. [DOI] [PubMed] [Google Scholar]
- 7.Peterman TA, Heffelfinger JD, Swint EB, Groseclose SL. The changing epidemiology of syphilis. Sex Transm Dis 2005;32(10 Suppl.):s4–s10. [DOI] [PubMed] [Google Scholar]
- 8.Eron JJ, Benoit SL, Jemsek J, et al. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N Engl J Med 1995;333:1662–1669. [DOI] [PubMed] [Google Scholar]
- 9.US Preventive Services Task Force (USPSTF). Screening for syphilis infection in nonpregnant adults and adolescents. JAMA 2016;315:2321–2327. [DOI] [PubMed] [Google Scholar]
- 10.Katz KA, Lee MA, Gray T, et al. Repeat syphilis among men who have sex with men—San Diego County, 2004–2009. Sex Transm Dis 2011;38:349–352. [DOI] [PubMed] [Google Scholar]
- 11.Jajosky R, Rey A, Park M, et al. Findings from the Council of State and Territorial Epidemiologists’ 2008 assessment of state reportable and nationally notifiable conditions in the United States and considerations for the future. J Public Health Manag Pract 2011;17:255–264. [DOI] [PubMed] [Google Scholar]
- 12.SAS (Statistical Analysis Software). SAS Institute Inc., Cary, NC. Available at: https://sas.com (Last accessed February 10, 2021). [Google Scholar]
- 13.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2021. Available at: https://www.R-project.org/ (Last accessed March 3, 2021). [Google Scholar]
- 14.RStudio Team. RStudio: Integrated development environment for R. Boston, MA: RStudio, PBC, 2021. Available at: www.rstudio.com/ (Last accessed March 5, 2021). [Google Scholar]
- 15.Wickham H ggplot2: Elegant graphics for data analysis. New York: Springer-Verlag, 2009. Available at: http://ggplot2.org (Last accessed March 11, 2021). [Google Scholar]
- 16.Kernai R, Lukehart S, Stenger M, et al. Is early latent syphilis more likely in patients with a prior syphilis infection? Paper presented at: 18th International Society for STD Research, London, United Kingdom, 2009. Abstract OS2.111.01. [Google Scholar]
- 17.Ogilvie GS, Taylor DL, Moniruzzaman A, et al. A population-based study of infectious syphilis rediagnosis in British Columbia, 1995–2005. Clin Infect Dis 2009;48: 1554–1558. [DOI] [PubMed] [Google Scholar]
- 18.Ciesielski C Repeat syphilis infection in MSM, 2000–2005. Paper presented at: 2006 National STD Prevention Conference, Jacksonville, FL, 2006. Abstract 375. [Google Scholar]
- 19.Phipps W, Kent C, Kohn R, et al. Risk factors for repeat syphilis in men who have sex with men, San Francisco. Sex Transm Dis 2009;36:331–335. [DOI] [PubMed] [Google Scholar]
- 20.Traegar MW, Schroeder SE, Wright EJ, et al. Effects of pre-exposure prophylaxis for the prevention of HIV infection on sexual risk behavior in men who have sex with men: A systematic review and meta-analysis. Clin Infect Dis 2018;67:676–686. [DOI] [PubMed] [Google Scholar]
- 21.Vuylsteke B, Reyniers T, De Baetselier I, et al. Daily and event-driven pre-exposure prophylaxis for men who have sex with men in Belgium: Results of a prospective cohort measuring adherence, sexual behaviour and STI incidence. J Int AIDS Soc 2019;22:e25407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoomenberg E, Coyer L, Achterbergh R, et al. Sexual behaviour and incidence of HIV and sexually transmitted infections among men who have sex with men using daily and event-driven pre-exposure prophylaxis in AMPrEP: 2 year results from a demonstration study. Lancet HIV 2019;6:e447–e455. [DOI] [PubMed] [Google Scholar]
- 23.Prescott MR, Hern J, Petersen M, Santos G-M. Does HIV pre-exposure prophylaxis modify the effect of partnership characteristics on condom use? A cross-sectional study of sexual partnerships among men who have sex with men in San Francisco, California. AIDS Patient Care STDS 2019; 33:167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramchandani MS, Golden MR. Confronting rising STIs in the era of PrEP and treatment as prevention. Curr HIV AIDS Rep 2019;16:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noiman A, Macalino G, Won SH, et al. Sexual risk behaviors associated with sexually transmitted infections in a US military population living with HIV after the repeal of “Don’t Ask, Don’t Tell.” AIDS Patent Care STDs 2020; 34:523–533. [DOI] [PubMed] [Google Scholar]
- 26.Horberg MA, Certa JM, Rubenstein KB, et al. Beyond the HIV care continuum and viral suppression: Broadening the scope of quality metrics for total HIV patient care. AIDS Patient Care STDs 2020;34:461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson DP, Prestage GP, Gray RT, et al. Chemoprophylaxis is likely to be acceptable and could mitigate syphilis epidemics among populations of gay men. Sex Transm Dis 2011;38:573–579. [DOI] [PubMed] [Google Scholar]
- 28.Bolan RK, Beymer MR, Weiss RE, et al. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: A randomized, controlled pilot study. Sex Transm Dis 2015;42:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunn RA, Klausner JD. Enhancing the control of syphilis among men who have sex with men by focusing on acute infectious primary syphilis and core transmission groups. Sex Transm Dis 2019;46:629–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grant JS, Stafylis C, Celum C, et al. Doxycycline prophylaxis for bacterial sexually transmitted infections. Clin Infect Dis 2020;70:1247–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]


