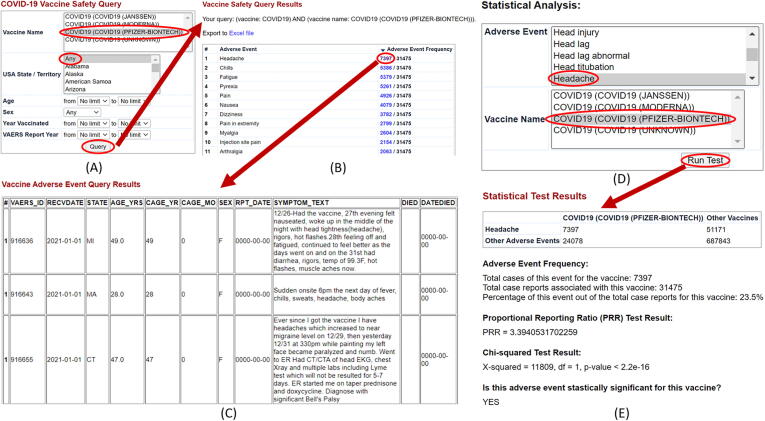
Fig. 3.
Cov19VaxKB adverse event query and statistical analysis tool. (A) A user can begin by selecting a specific vaccine and specifying various filters including US state/territory, age, sex, year vaccinated, and VAERS report year. (B) After the user clicks “Query,” the query will generate a list of adverse events with their frequency of case reports. (C) The user can click an adverse event frequency to view a detailed table of individual VAERS case reports. (D) To perform statistical analyses, the user can select an adverse event and specify a vaccine of interest. (E) After clicking “Run Test,” the user will be presented with a formatted 2x2 table of AE case report counts and statistical test results, including a Chi-squared analysis, PRR, and statistical significance result.