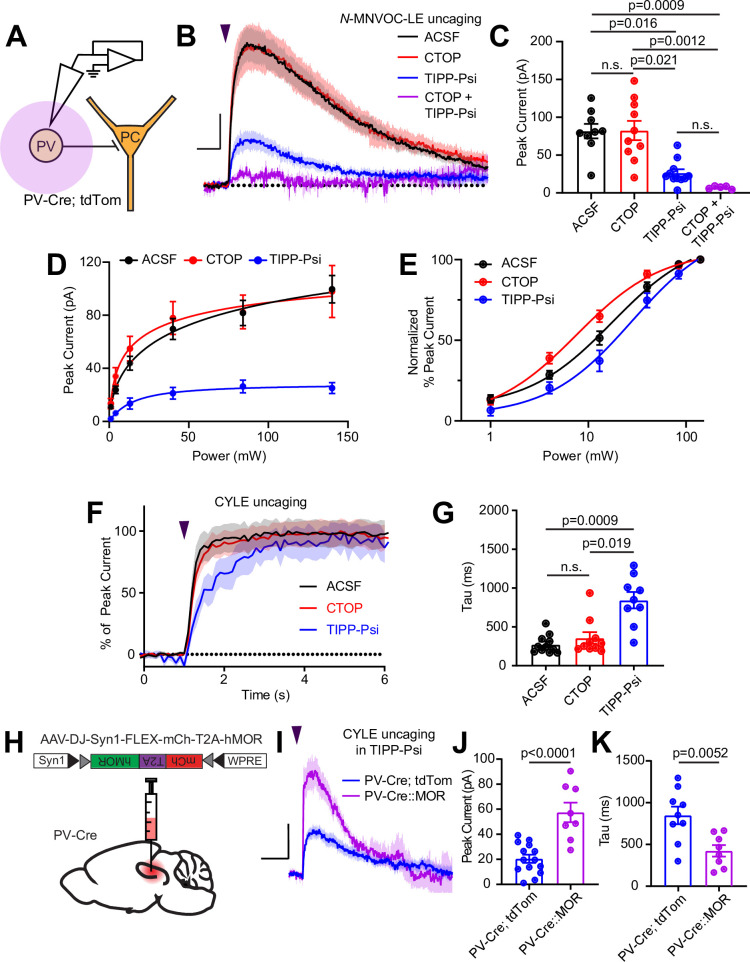
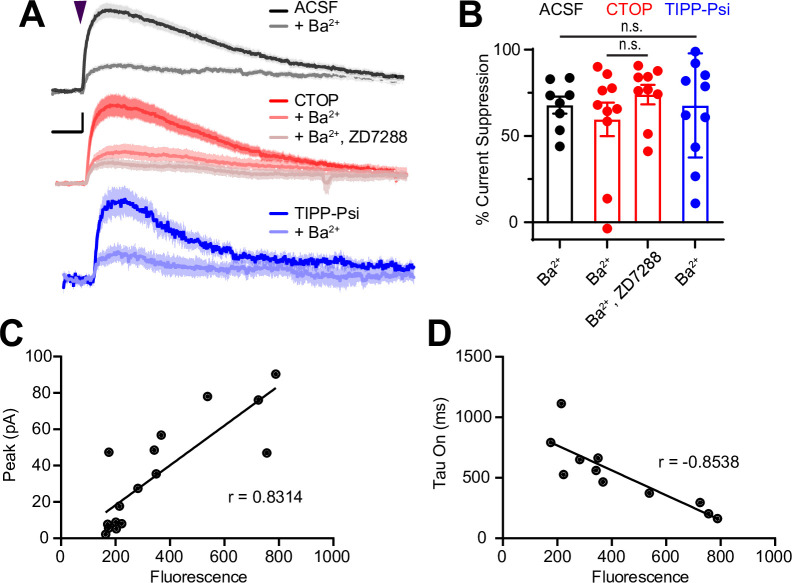
Figure 4. Enkephalin evokes outward currents in CA1 parvalbumin (PV) interneurons through both mu and delta opioid receptors.
(A) Schematic of whole-cell voltage clamp recording configuration from PV interneurons with peptide uncaging. (B) Average outward currents evoked by photoactivation of N-MNVOC-LE (6 μM) with an 84 mW light flash in the absence (black, artificial cerebrospinal fluid [ACSF], n = 9 cells from five mice) and presence of mu and delta opioid receptor antagonists (red, CTOP, n = 10 cells from six mice; blue, TIPP-Psi, n = 11 cells from six mice; purple, CTOP+ TIPP-Psi, n = 5 cells from three mice). Scale bar: x = 5 s, y = 20 pA. (C) Summary of peak current amplitudes shown in B. (D) Linear optical power-response curve of peak current as a function of light intensity, in the absence (ACSF, black, n = 9 cells per laser intensity) and presence of either CTOP (red, n = 10 cells) or TIPP-Psi (blue, n = 11 cells). (E) Logarithmic optical power-response curves of the data in D normalized to the maximal peak current observed in each condition. (F) Rising phase of the average peak-normalized outward currents evoked by photoactivation of CYLE (6 μM) with an 84 mW light flash in the absence (black, ACSF, n = 11 cells from four mice) and presence of mu and delta opioid receptor antagonists (red, CTOP, n = 10 cells from four mice; blue, TIPP-Psi, n = 12 cells from four mice). (G) Time constants of current activation in response to photoactivation of CYLE from F. (H) Schematic of viral Cre-dependent mu opioid receptor over-expression in CA1 of PV-Cre mice. (I) Average outward currents evoked by photoactivation of CYLE by an 84 mW light flash in the presence of TIPP-Psi in either PV-Cre; tdTom mice (blue, data from B) or PV-Cre mice overexpressing the mu opioid receptor (purple, n = 8 cells from three mice). Scale bar: x = 10 s, y = 20 pA. (J) Summary of current amplitudes shown in I. (K) Time constants of current activation in response to photoactivation of CYLE.