Figure 7.
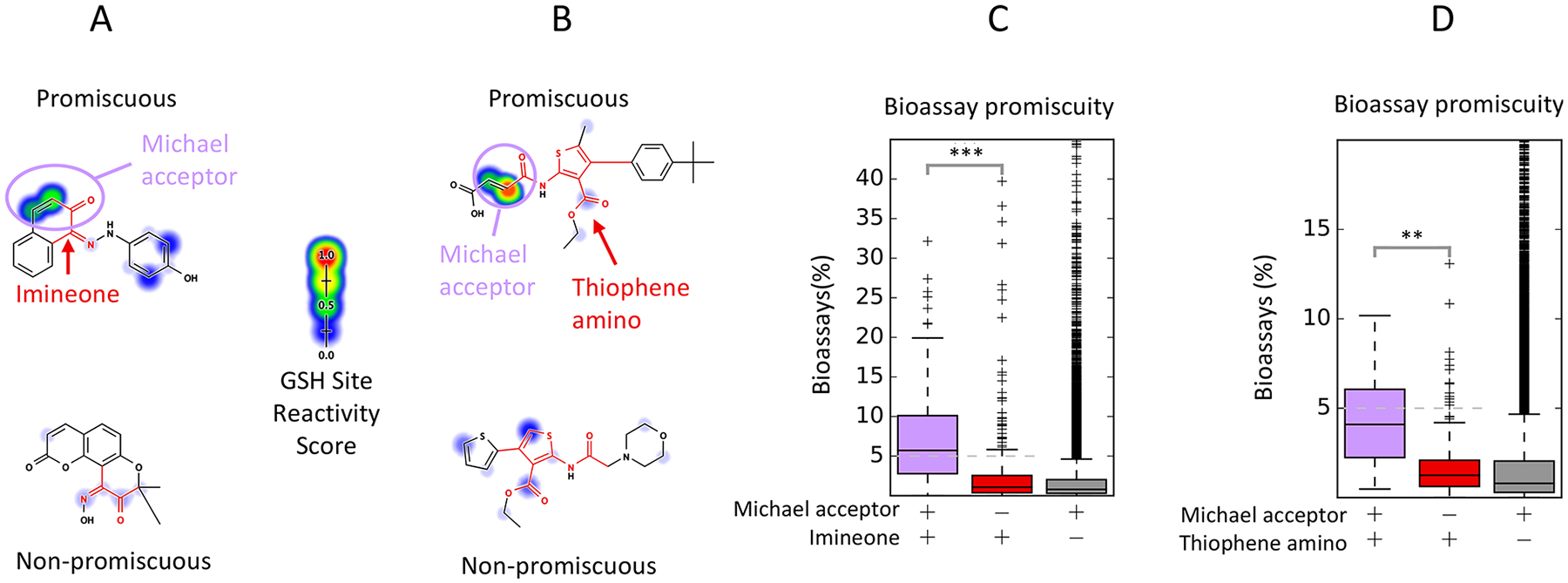
Many PAINS filters are associated with a nearby or overlapping reactive Michael-acceptor motif. (A) The imineone filter group matches a chemical motif with adjacent imine and ketone groups, as well as diones. Among 457 compounds matching this filter (red), 185 compounds (40%) possess a Michael-acceptor motif that overlaps with the motif matched by this filter group (purple). Michael-acceptor motifs are well-known electrophiles assigned high reactivity scores by our model.71 (B) The thiophene_amino filters match various substituted thiophene rings. Among 224 compounds matching this filter group, 28 (13%) contain a Michael-acceptor motif adjacent to the amide and outside the motif matched by the filter group (purple). (C, D) Compounds with this Michael-acceptor are enriched 3.99-fold for promiscuous actives among compounds matching the imineone filter (p < 10−10, χ2 test), while compounds matching the thiophene amino filter group and the Michael-acceptor motif are enriched 3.30-fold for promiscuous actives (p = 7.97 × 10−3, χ2 test). Compounds with Michael-acceptor motifs not matching the imineone or thiophene amino filters are not strongly enriched for promiscuous bioactivity. **: p < 0.001, ***: p < 0.0001.
