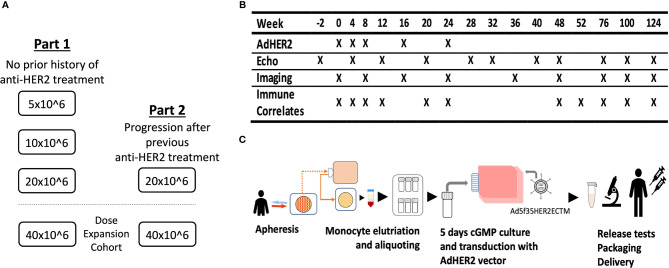
Figure 1.
The clinical trial design of the AdHER2 DC vaccine. (A) Part 1 with dose escalation was opened to enroll 1) patients with metastatic cancer that progressed after at least one standard therapy or 2) with high-risk bladder cancer who completed treatment with curative intent and no radiographic evidence of disease. After reviewing the safety data of part 1, part 2 and dose expansion cohorts followed. (B) Study calendar showing schedules for vaccination and assessments. (C) Diagram showing AdHER2 DC vaccine manufacturing. Briefly, mononuclear cells of patients were collected by apheresis and elutriated monocyte aliquots were stored frozen until each vaccine dose was manufactured. On day 0, one aliquot was thawed and resuspended in media containing cytokine and plasma. On day 2, the medium was changed and keyhole limpet hemocyanin was added as an immune adjuvant. On day 3, cells were transduced with the AdHER2 vector designed to express the ECTM of HER2. Then, the maturation cocktail was added. On day 4, the product was reviewed and packaged for administration.