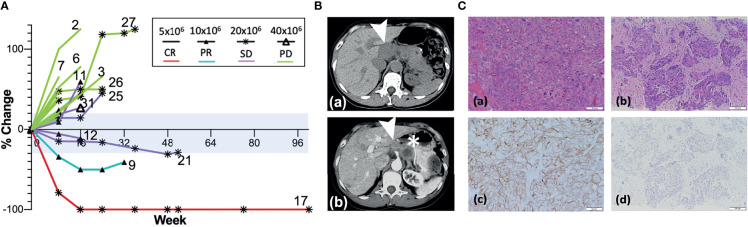
Figure 2.
Responses after AdHER2 DC vaccination. (A) Best responses in evaluable patients. Note the durable response in patients 21 and 17 with non-target lesion progressions at the time of progression while their target lesions were still showing responses. Some patients were determined as having PD because non-target lesion progression was noted as shown in patient 12 who showed SD initially at week 8 but determined as PD at week 16 despite original target lesions remained in the range of SD. The range for a stable disease (SD, −30% to +20% change in the sum of target lesions) is tinted with light blue. Not all patients who progressed at week 8 were labeled with the patient number in the figure. The summary of the response type and duration can be found in Table 3 . (B) CT scans of patient 9 at baseline (a) and at week 24 (b). The target lesion (➤, 4.7 × 1.9 cm) at baseline decreased to 1.9 × 1.9 cm at week 24, but a non-target lesion (∗) progressed. (C) Microscopic exam of tumor tissue (×400) from patient 17; (a, b) H–E staining; (c, d) IHC of HER2; (a, c) oophorectomy specimen at the time of diagnosis; (b, d) at the time of recurrence showing high-grade serous ovarian cancer with HER2 3+ at diagnosis. (d) IHC of HER2 showing the absence of HER2 expression at the time of recurrence is suggestive of immune escape.