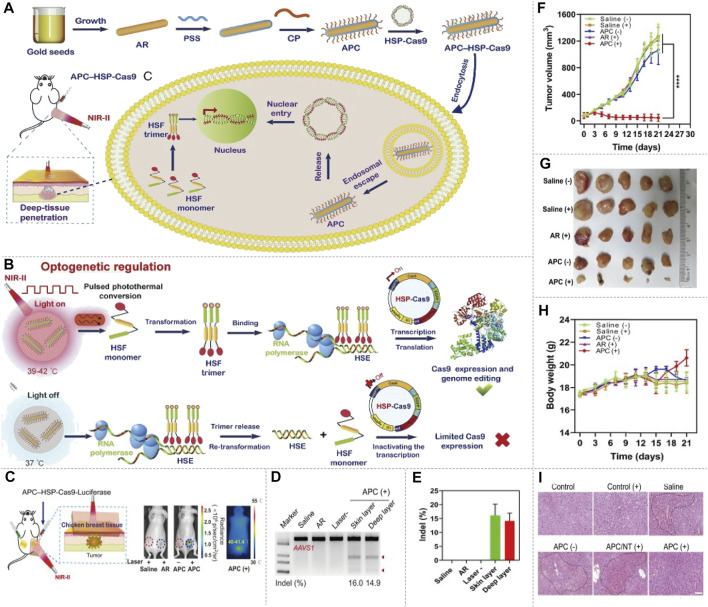
FIGURE 6.
NIR-II/CRISPR-Cas9 system optogenetic regulation of genome editing. (A) The schematic of preparation of the APC–HSP complex, delivery of APC–HSP-Cas9 complexes and deep-tissue penetration. The APC–HSP-Cas9 complex is composed of a cationic polymer-coated Au nanorod (APC) and the Cas9 plasmid driven by a heat-inducible HSP70 promoter. APC plasmid is internalized by the targeted cell through endocytosis. The Au nanorod serves as a photothermal transducer to transform the NIR light into intracellular local heat to trigger the transcription of Cas9 and sgRNA. (B) The mechanism of inducible optogenetic regulation of Cas9-mediated genome editing. Upon NIR light irradiation, APC quickly generates localized heat, which induce the transformation of the heat-shock factor (HSF) from monomers to trimers. The HSF trimers bind with the heat-shock element (HSE) of the HSP70 promoter results in the transcription of Cas9. Once the light irradiation is switched off, the bound trimer is released from the HSE and back to monomers to inactivate the transcription process. (C) Tumor-bearing mice are administered APC–HSP-Cas9 complexes through peritumoral injection, and the tumor is then exposed to irradiation for 30 min in the presence of breast chicken tissue (5-mm thickness) covering the tumor position to simulate the deep-tissue condition, the luciferase expression shows optogenetic genome editing could be manipulated in the deep tissue of local lesions. (D) Indel mutations detected by T7E1 assay. A significant mutation is detected from both the surface and deep layer of the tumor tissues. (E) Quantitative analysis of indel mutations. The indel rate of the surface and deep layer of the tumor tissues is 16.0 and 14.9%, respectively. (F) Tumor growth curve after the transfection of APC–HSP-Cas9 complexes, followed by NIR light irradiation. The tumorbearing mice injected with APC HSP-Cas9 targeting Plk1 exhibit significant tumor regression under irradiation. (G) Images of tumor tissues with different treatments. (H) The body-weight change during the treatment. A slight increase in body weight is observed at the end of the APC HSP-Cas9 targeting Plk1 after irradiation treatment. (I) H&E staining of liver slices from mice 10 days after the treatment. The mice treated with galactose-modified APC HSP-Cas9 Fas significantly reduce hyperemia, shows the APC treatment merely induce any liver toxicity (reproduced from (Chen X. et al., 2020) with permission from Proceedings of the National Academy of Sciences of the United States of America).