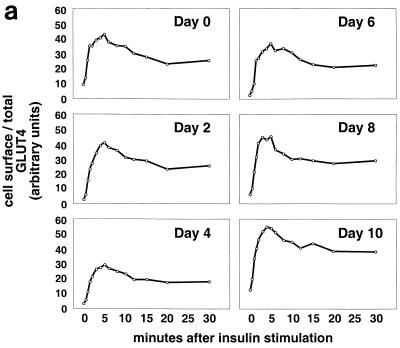
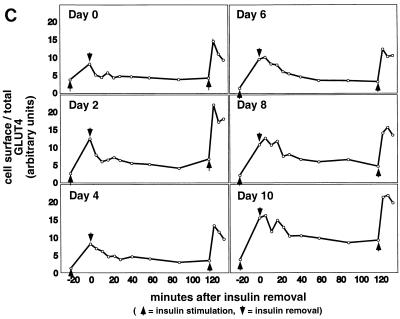
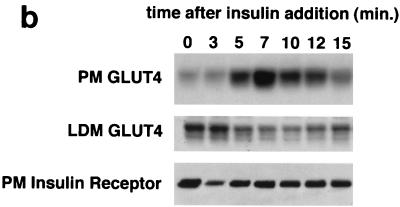
FIG. 3.
Kinetics of GLUT4 trafficking in 3T3-L1 cells. (a) Confluent 3T3-L1 preadipocytes (day 0) or 3T3-L1 cells at various stages of adipocyte differentiation (as indicated) were treated with insulin for various lengths of time, and changes in the proportion of GLUT4 reporter present at the cell surface were analyzed. Data are plotted for basal cells and for cells treated with 80 nM insulin for 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 10, 12, 15, 20, or 30 min. Membrane trafficking was stopped by washing with cold PBS, cells were stained at 4°C for externalized Myc epitope tag using a PE-conjugated secondary antibody, and PE and GFP fluorescence intensities were measured using flow cytometry as described in the text. Regardless of the state of differentiation, insulin causes a rapid externalization of GLUT4 that peaks 4 to 5 min after insulin addition. Subsequently, there is a net internalization, so that a steady state in the presence of insulin is reached 20 min after insulin addition. The numbering on the vertical scale indicates a relative measure of GLUT4 at the cell surface, and these arbitrary units cannot be compared in absolute terms to those in other figures. (b) Insulin-stimulated translocation of endogenous GLUT4 to the plasma membrane of 3T3-L1 adipocytes was analyzed by subcellular fractionation. Cells were starved overnight and then stimulated with 480 nM insulin (added from a prewarmed, 3× stock) for various amounts of time. Cells were transferred to 4°C, washed with cold PBS, and fractionated as described in Materials and Methods. Plasma membrane (PM) and low-density microsomal (LDM) fractions were analyzed by immunoblotting. Equal amounts of protein were loaded in each lane. GLUT4 translocates to the plasma membrane in a biphasic pattern, with a peak at 7 min after insulin addition, followed by a subsequent decrease. A reciprocal pattern is observed in the LDM fraction. As a control, the PM immunoblot was reprobed with an anti-insulin receptor (β-subunit) antibody, which demonstrates similar amounts of insulin receptor at the plasma membrane at all time points. The experiment was performed twice, with similar results each time. (c) 3T3-L1 preadipocytes (day 0) or cells at various times during adipocyte differentiation were stimulated with 80 nM insulin for 20 min, then placed at 4°C, and washed with an acidic buffer to remove insulin. Cells were rewarmed in serum-free medium to allow GLUT4 reinternalization for 6, 12, 18, 24, 30, 40, 60, 90, or 120 min; some cells that had been rewarmed for 120 min were restimulated with 80 nM insulin for 5, 10, or 15 min. Cells were stained for cell surface Myc epitope and analyzed by flow cytometry as described in the text. In all instances, the GLUT4 reporter was reinternalized after removal of insulin and recycles upon readdition of insulin.