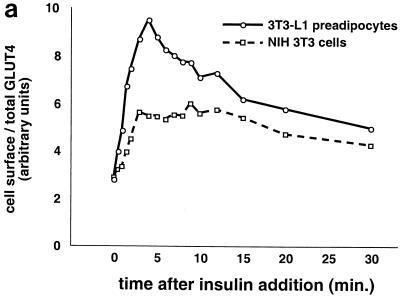
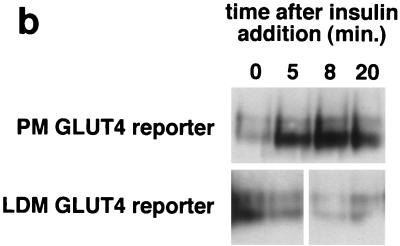
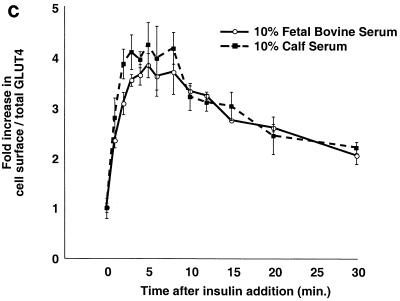
FIG. 4.
Kinetics of GLUT4 trafficking in 3T3-L1 preadipocytes and NIH 3T3 cells. (a) Confluent 3T3-L1 preadipocytes and NIH 3T3 cells expressing similar amounts of the reporter were treated with 160 nM insulin for various lengths of time, chilled, and analyzed by FACS to measure changes in the proportion of GLUT4 reporter present at the cell surface. Insulin caused a much more marked redistribution of GLUT4 to the plasma membrane of 3T3-L1 preadipocytes than of NIH 3T3 cells. (b) Translocation of the GLUT4 reporter in 3T3-L1 preadipocytes was assayed by subcellular fractionation and immunoblotting. After serum starvation, cells were stimulated with 480 nM insulin (added from a prewarmed 3× stock) for various amounts of time, then washed with cold PBS++, and fractionated as described in Materials and Methods. Equal amounts of protein were loaded in each lane. Insulin caused an increase in the amount of GLUT4 reporter in the plasma membrane (PM) fraction, with a peak response at 8 min after insulin addition and a subsequent decrease at 20 min. A reciprocal pattern is apparent in the low-density microsomal (LDM) fraction. The experiment was performed twice, with similar results each time. (c) Confluent 3T3-L1 preadipocytes were cultured for 3 days in either 10% fetal bovine serum or 10% calf serum. Cells were then starved, stimulated with 160 nM insulin for various amounts of time, and analyzed by flow cytometry. Samples were measured in duplicate (control samples were in triplicate or quadruplicate). Insulin caused similar increases in the fraction of GLUT4 reporter at the plasma membrane under both conditions. The experiment was done twice, with similar results each time.