Figure 1.
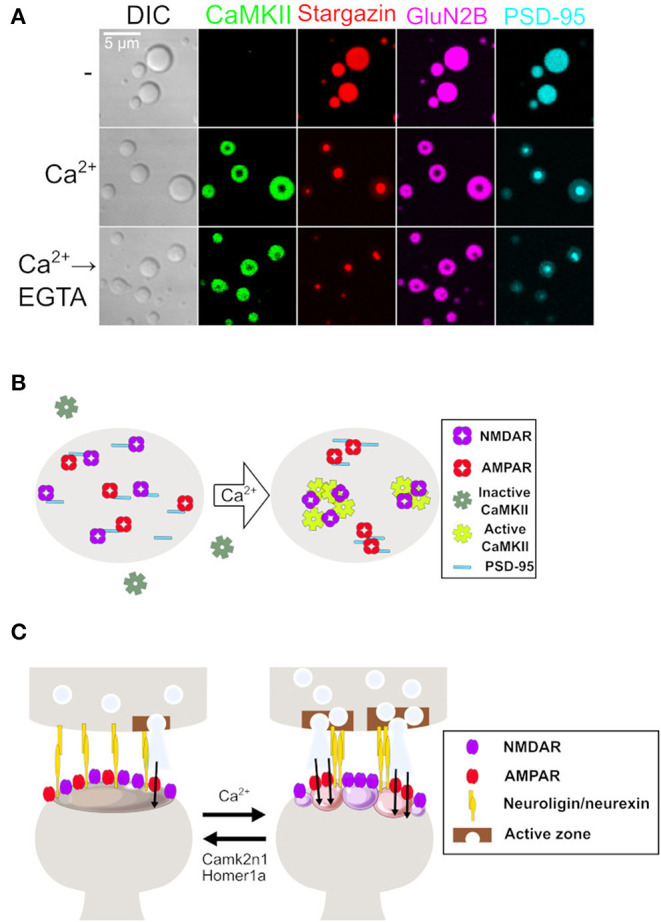
LLPS to synaptic plasticity. (A) Microscope images of protein condensates consist of the following four proteins during calcium stimulation. From left to right, confocal images of differential interference contrast and confocal fluorescent images of CaMKII, Stargazin, GluN2B, and PSD-95. Stargazin, GluN2B, and PSD-95 are homogenously distributed before Ca2+. Ca2+ triggers the incorporation of CaMKII and the formation of a nanodomain-like structure inside of condensate. The condensate is sustained even after the removal of Ca2+ by ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA). Modified from Hosokawa et al. (2021). (B) An end view of the postsynaptic membrane. Receptors and PSD proteins in naïve synapses are evenly distributed. Ca2+ influx triggers persistent sub-compartmentalization of proteins into the Stargazin-PSD-95 group and CaMKII-GluN2B group, resulting in the formation of the AMPAR nanodomain. Modified from Hosokawa et al. (2021). (C) Side sectional view of the synapse. Ca2+ influx triggers the formation of AMPAR nanodomains via PSD clustering and LLPS. This affects protein assembly in the presynaptic terminal through adhesion proteins as a retrograde signal. In contrast, a dissociation of LLPS by Camk2n1 and Homer1a might act as a mechanism to depress synaptic strength by disrupting the AMPAR nanodomain and PSD clustering. Modified from Hosokawa et al. (2021). LLPS, liquid-liquid phase separation; PSD, postsynaptic density; AMPAR, α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate-type glutamate receptor.
