Abstract
Background
Due to the constraints surrounding autograft bone, surgeons have turned to osteoinductive agents to augment spinal fusion. Reports of complications and questionable efficacy slowed the adoption of these alternatives. Recombinant human platelet‐derived growth factor B homodimer (rhPDGF‐BB) has been Food and Drug Administration (FDA)‐approved (Augment) to promote fusion in other areas of orthopedics, but its characterization in spine fusion has not yet been tested. The purpose of this study is to characterize the host response to PDGF‐BB in vivo.
Methods
Eighty female Fischer rats underwent L4‐5 posterolateral fusion using one of four implant types: (a) iliac crest syngeneic allograft harvested from syngeneic donors, (b) β‐TCP/bovine collagen matrix (β‐TCP/Col) with sodium acetate buffer, (c) β‐TCP/Col with 0.3 mg/mL “low dose,” or (d) β‐TCP/Col with 3.0 mg/mL “high dose” of rhPDGF‐BB. Animals underwent magnetic resonance imaging (MRI) and serum cytokine quantification at 4, 7, 10, and 21 days, postoperatively. Tissues were processed for immunofluorescence staining for Ki67 and von Willebrand factor (vWF) to assess neovascularization.
Results
MRI demonstrated no differences in fluid accumulation among the four treatment groups at any of the time points. Serum cytokine analysis showed no clinically significant differences between treatment groups in 20 of the 27 cytokines. Inflammatory cytokines IFN‐γ, IL‐1β, IL‐18, MCP‐1, MIP‐1α, TNF‐α were not induced by rhPDGF‐BB. Histology showed no differences in cell infiltration, and Ki67 and vWF immunofluorescence staining was similar among groups.
Conclusions
rhPDGF‐BB delivered with a β‐TCP/Col matrix exerts no exaggerated systemic or local host inflammatory response when compared to iliac crest syngeneic allograft bone or the control carrier. rhPDGF‐BB mixed with a β‐TCP/Col matrix could be a viable and safe biologic alternative to syngeneic allograft in spine fusion. Further studies need to be performed to evaluate efficacy in this setting.
Keywords: arthrodesis, biologic, cytokine, MRI, PDGF, platelet‐derived growth factor, spine surgery
Recombinant human platelet‐derived growth factor B homodimer (rhPDGF‐BB) has been FDA‐approved (AUGMENT) to promote fusion in other areas of orthopedics, but its characterization in spine fusion has not yet been tested. Our study demonstrated that rhPDGF‐BB delivered with a β‐TCP/Col matrix exerts no exaggerated systemic or local host inflammatory response when compared to iliac crest autograft bone or the control carrier. rhPDGF‐BB mixed with a β‐TCP/Col matrix could be a viable and safe biologic alternative to autograft in spine fusion.
1. INTRODUCTION
Augmentation of the body's natural capacity for bone regeneration is critical for many orthopedic procedures such as fracture repair, osteotomies, and spine fusion. Spine arthrodesis is frequently performed in the treatment of spine trauma, deformity, and complex degenerative disorders. With an estimated 413 000 fusion procedures performed in the United States annually, the number of procedures performed has increased by 2.4‐fold since 1998. 1 As the incidence of spine fusion procedures increases, the demand for safe yet effective biologics to improve fusion rates has risen dramatically.
Iliac crest autograft is regarded as the gold standard for posterolateral lumbar spine fusion. However, it is now rarely used in practice because of surgeon and patient concerns of morbidity such as donor site pain, wound complications, and increased operative time. 2 , 3 Novel spine biologics could allow for the improvement of fusion rates, pain reduction, and clinical outcomes while avoiding the complications associated with autograft. However, some of these alternatives have led to complications associated with the heightened local inflammatory response. 4 , 5 , 6 , 7 , 8 Post‐FDA approval of INFUSE, preclinical studies evaluating the use of high‐dose rhBMP‐2 in the setting of spine fusion revealed a significant systemic cytokine response that we associated with the side effects seen with clinical use of the growth factor. 9
Platelet‐derived growth factor‐B chain homodimer (PDGF‐BB) is a polypeptide growth factor released from platelets that acts early in the wound healing cascade as a chemoattractant and mitogenic agent for osteoprogenitor cells and differentiated osteoblasts. 10 , 11 , 12 These properties make PDGF‐BB vital in promoting healing at sites of bone fusion. Recombinant human PDGF‐BB combined with an osteoconductive, bioresorbable synthetic bone matrix consisting of β‐tricalcium phosphate (β‐TCP) particles and bovine type I collagen (Col) in preclinical studies was shown to induce more bone formation in both in vivo osteoporotic and diabetic models as well as during distraction osteogenesis. 13 , 14 , 15 This bone graft substitute is currently FDA‐approved for use as an alternative to autograft in arthrodesis of the ankle and hindfoot (Augment) 16 , 17 and periodontal osteogenesis (GEM 21S). 18 From the clinical studies in foot arthrodesis and in an ovine interbody fusion model, rhPDGF‐BB with an osteoconductive matrix has shown similar fusion outcomes compared to autograft without the complications of autograft harvest. 17 , 19 , 20
The use of rhPDGF‐BB combined with β‐TCP as a bone graft substitute in spine fusion has been proposed as a possible alternative to currently available options, but its use has not yet been evaluated for safety or efficacy in the spine. Given the association of PDGF‐BB with the neuroinflammatory cascade, 21 , 22 its impact combined with β‐TCP must be understood before considering its application for spinal fusion. This study sought to evaluate the host response following paraspinal intramuscular implantation of rhPDGF‐BB combined with a β‐TCP/Col matrix in a rat model.
2. MATERIALS AND METHODS
2.1. Study design
This study was sponsored by the Wright Medical Group, Inc. Institutional Animal Care and Use Committee approval at Northwestern University was obtained under protocol number IS00008387 and with Animal Assurance number D16‐00182 (A3283‐01) with the Office of Laboratory Animal Welfare. Eighty female Fischer F344 rats age 12 to 16 weeks old underwent L4‐5 intertransverse lumbar spinal fusion using one of the following four adjuncts: (a) iliac crest allograft from syngeneic donors, referred herein as “allograft” as a surrogate for autograft (n = 20); (b) vehicle control (sodium acetate/β‐TCP/Col matrix, “matrix alone”) (n = 20); (c) low‐dose (0.3 mg/mL) rhPDGF‐BB/matrix (n = 20); or (d) high‐dose (3.0 mg/mL) rhPDGF‐BB/matrix (n = 20) (Table 1). The PDGF doses were selected based on previous studies which used concentrations of either 0.3 or 1.0 mg/mL, 17 the animal model used, and the current dose of rhPDGF that is used clinically in ankle arthrodesis. The relative maximum dose of rhPDGF‐BB during clinical use has been recommended to be 39 μg rhPDGF‐BB/kg (~2700 μg rhPDGF‐BB/70 kg person, given max volume of 9 mL of 0.3 mg/mL solution). 17 The chosen maximum dose used in this study was approximately 6000 μg rhPDGF‐BB/kg, which was designed to exceed the clinical dose by 150‐fold to highlight any potential adverse effects. Animals in the allograft group received 0.4 cm3 of fresh morselized syngeneic donor iliac crest bone bilaterally.
TABLE 1.
Treatment group and time points
| Treatment group | 4 days | 7 days | 10 days | 21 days | Total |
|---|---|---|---|---|---|
| Allograft | 5 | 5 | 5 | 5 | 20 |
| Matrix alone | 5 | 5 | 5 | 5 | 20 |
| Low‐dose PDGF (0.3 mg/mL rhPDGF‐BB) | 5 | 5 | 5 | 5 | 20 |
| High‐dose PDGF (3.0 mg/mL rhPDGF‐BB) | 5 | 5 | 5 | 5 | 20 |
2.2. Test article description and preparation
Test articles included vehicle (or “matrix alone”) (sodium acetate buffer only, 20 mM, pH 6.0), low‐dose rhPDGF‐BB (0.3 mg/mL) in sodium acetate buffer, and high‐dose rhPDGF‐BB (3.0 mg/mL) in sodium acetate buffer combined with an osteoconductive matrix composed of β‐TCP particles (100‐300 μm in diameter) and bovine type I collagen in an 80:20 ratio. Prior to implantation, 1 mL of test solution was added to 0.5 g of the test article matrix in a 10‐mL syringe and incubated at room temperature for 2 minutes to hydrate the matrix. The test article was mixed using a female‐to‐female luer lock connector and by transferring the contents between the two syringes to create a paste, and was then backfilled into a sterile syringe for transfer and implantation. During surgery, the animals received 0.26 cm3 of the test article at L4‐L5 bilaterally.
2.3. Surgical technique
Rats were maintained under continuous isoflurane anesthesia throughout the procedure. Buprenex SR (0.03 mg/kg) and meloxicam (1 mg/kg) were administered subcutaneously immediately prior to surgery for pain control. Using a previously described surgical technique, 9 separate fascial incisions were made 4 mm from the midline exposing the L4 and L5 transverse processes. The overlying lumbar paraspinal muscular compartment was resected en bloc bilaterally to create a dead space adjacent to the spine (Figure 1). Paraspinal muscle tissue resections averaged 1.047 ± 0.097 g. After visualization of the L4 and L5 transverse processes, gentamicin (1.0 mg/mL) was applied to the fusion bed for 10 to 20 seconds and then removed. After decortication with a high‐speed burr, the graft materials were implanted between the L4 and L5 transverse processes. Fascia was closed bilaterally with use of a 3‐0 monofilament poliglecaprone 25 absorbable interrupted suture (Monocryl; Ethicon, Inc., Somerville, NJ). Skin was closed using a nonabsorbable 3‐0 nylon suture in an interrupted fashion (Ethilon; Ethicon, Inc., Somerville, NJ). After wound closure, 5 mL of Lactated Ringer solution was administered intraperitoneally and the animals were returned to a cage on a heating pad. Animals were monitored continuously until ambulatory and then every 15 minutes for the first hour after surgery, and they were allowed to eat, drink, and bear weight ad libitum. Meloxicam (0.5 mg/kg) was administered every 24 hours for 3 days.
FIGURE 1.
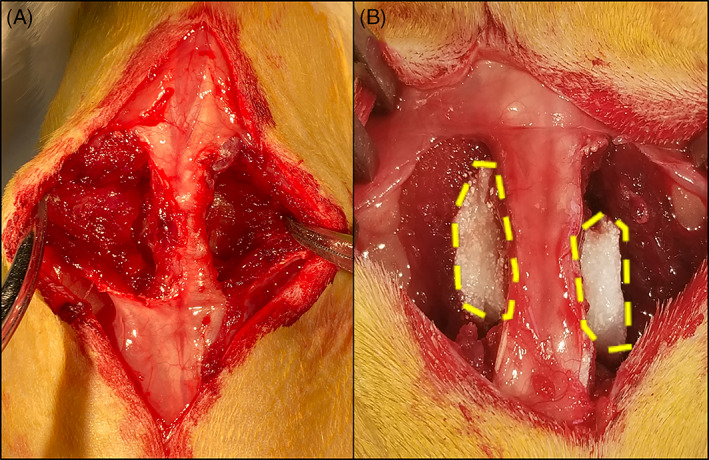
Modified rat arthrodesis model with resected tissue demonstrating (A) the exposure of the lumbar transverse process and fusion bed and (B) the implanted test article with resected paraspinal muscles. The implanted test article is outlined by the yellow dashed lines
2.4. Magnetic resonance imaging and analysis
At 4, 7, 10, and 21 days postoperatively, five animals in each treatment group were euthanized with exsanguination using a bilateral thoracotomy technique. Animals were scanned immediately after euthanasia. All animals were imaged in a 7 T Clinscan MRI scanner (Bruker, Ettlingen, Germany) using a quadrature volume transceiver coil. The imaging protocol included a coronal T2‐weighted turbo spin echo (TSE) sequence (TR/TE = 5310/62 ms, slice thickness = 1.2 mm, voxel size = 0.18 × 0.18 mm2, matrix size = 384 × 384, number of slices = 34) and a sagittal T2‐weighted TSE (TR/TE = 5310/62 ms, slice thickness = 1.2 mm, voxel size = 0.20 × 0.20 mm2, matrix size = 270 × 384, number of slices = 34). These two sequences were used to localize the area between the L4‐5 disc to position a transversal T2‐weighted TSE (TR/TE = 5940/62 ms, slice thickness = 0.9 mm, voxel size = 0.18 × 0.18 mm2, matrix size = 384 × 306, number of slices = 38). A water reference was placed under the body of each rat and acquired simultaneously with the animal.
The transverse MR images were normalized to water references using Xinapse Jim Version 8.0 software (Xinapse Systems, Ltd, Essex, UK). Representative axial slices at the L4‐5 disc space (midpoint within the fusion bed) were identified. After processing, the normalized images were used for the segmentation analysis using ITK‐SNAP version 3.0 (www.itksnap.org), 23 an open‐source MR analysis software. After adjusting for appropriate contrast to visualize the sample, the lumbar region of interest was manually selected using a polygon tool to highlight the region between L2‐L3 cranially and L5‐L6 caudally. These were chosen in order to (a) ensure interspecimen reproducibility and (b) capture the fluid accumulation adjacent to the fusion bed. To minimize the confounding influence of other hyperintense areas, we removed the spinal column and skin injury from the region of interest. The semi‐automated algorithm from ITK‐SNAP used threshold‐based contouring to isolate the areas of hyperintensity within the selected region of interest. The threshold values were set to eliminate areas of hypointensity (ie, air, bone). The volume of the hyperintense signal within the threshold was recorded for each animal as a surrogate for fluid collection and indicator of inflammatory response.
2.5. Serum cytokine analysis
During euthanasia, exsanguination was achieved using a bilateral thoracotomy technique with intracardiac puncture to collect whole blood. Samples were incubated at room temperature for 10 minutes, then centrifuged at 13 200g for 10 minutes. Serum supernatant was removed and stored at −80°C until analysis. A multiplex bead‐based Luminex assay (Luminex Corp, Austin, TX or Milliplex MAP [EMD Millipore, Billerica, MA]) was used to quantify a panel of 27 cytokines in the rat sera (Table 2). The experiment was run in duplicate for each sample. xMAP technology was used to quantify the fluorescence and subsequently concentration (pg/mL) of each analyte using individual standard curves. On numerous occasions, several, but not all of the values of a given analyte within a treatment group/time point were below the minimum detectable concentration (minDC) for that analyte. In such a case, that particular sample was assigned the minDC. Cytokine levels were reported as calculated pg/mL. IFN‐γ, IL‐1β, IL‐18, MCP‐1, MIP‐1α, TNF‐α were selected based on our previous model and presented as a comparative fold change.
TABLE 2.
Serum cytokines analyzed by Luminex xMAP technology
| Serum cytokines | ||
|---|---|---|
| EGF | IL‐2 | IP‐10 |
| Eotaxin | IL‐4 | Leptin |
| Fractalkine | IL‐5 | LIX |
| G‐CSF | IL‐6 | MCP‐1 |
| GM‐CSF | IL‐10 | MIP‐1α |
| GRO/KC/CINC‐1 | IL‐12p70 | MIP‐2 |
| IFNγ | IL‐13 | RANTES |
| IL‐1α | IL‐17A | TNFα |
| IL‐1β | IL‐18 | VEGF |
Abbreviations: EGF, epidermal growth factor; G‐CSF, granulocyte‐colony stimulating factor; GM‐CSF, granulocyte‐macrophage colony stimulating factor; GRO/KC/CINC‐1, growth regulated oncogene/keratinocyte chemoattractant/cytokine‐induced neutrophil chemoattractant; IFN, interferon; IL, interleukin; IP, interferon γ‐induced protein; LIX, liposaccharide‐induced CXC chemokine; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normal T‐cell expressed, and secreted; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
2.6. Histology
Spine specimens were resected en bloc from the L2 to L6 with the paraspinal muscles intact. The specimens were fixed for 24 hours in 10% neutral buffered formalin and then stored in 70% ethanol. The samples were then decalcified in a 5% formic acid solution and subsequently embedded in paraffin. Serial sagittal 5 to 6 μm slices were made along the transverse processes of L4 and L5. The slides were stained with hematoxylin and eosin.
2.7. Immunofluorescence staining and analysis
Paraffin‐embedded spine specimens were sectioned with serial sagittal 5 to 6 μm slices along the transverse processes of L4 and L5. Formalin‐fixed slides were placed in a 60°C oven to melt wax and then deparraffinized and rehydrated through a xylene and ethanol ramp. Epitopes were then unmasked through heat‐induced epitope retrieval in a citrate buffer (pH 6.0). Slices were single‐stained with the following primary antibodies: (a) rabbit anti‐Ki67 antibody (ab15580, Abcam) in 1:200 dilution; (b) rabbit anti‐vWF antibody (ab6994, Abcam) in 1:200 dilution. A goat anti‐rabbit FITC‐labeled antibody was used as a secondary antibody (ab6717, Abcam) in 1:500 dilution. The primary antibodies were incubated at 4°C overnight and the secondary antibodies were incubated at room temperature for 1 hour. Slices were counterstained with DAPI nuclear stain on an Intellipath Automated staining platform (BioCare) in the Mouse Histology and Phenotyping Laboratory core facility. The entirety of the tissue slices was imaged with a TissueGnostic histological microscope (Zeiss), utilizing a 10× objective, and fixed acquisition parameters (ie, lamp intensity, exposure time). For each slide, all of the acquired 10× images were automatically stitched together (50% overlap) to obtain a large image rendering. The fluorescent signal was thresholded through the image analysis software NIS Elements (Nikon). Two region of interests (ROIs) were then drawn, one per side of the spine, and placed between L4 and L5, where the scaffolds were placed (Figure S1, Supporting Information). The two ROIs were the same size and were maintained the same throughout the analysis of all specimens, to ensure consistency in the number of positive pixels measured in every image. The ROIs were automatically applied to each image by the analysis software, and their position on the image was manually corrected to ensure optimal placement before the analysis. The area with pixels positive for Ki67 or vWF within the ROIs was automatically measured and the resulting “binary area” (μm2) was used to determine differences in the expression of the selected markers over time, among treatment groups.
2.8. Statistical analysis
A power analysis performed using previously collected data set showed that a sample size of 5 at each time point was sufficient to detect statistical significance with α = .05 and 80% power. 9 IBM SPSS Statistics version 24 (IBM Corp, Armonk, NY) was used to perform all statistics. Differences were determined by one‐way analysis of variance (ANOVA) with Tukey's post hoc test (cytokine analysis) or Welch's ANOVA with Games‐Howell post hoc test (magnetic resonance imaging [MRI] analysis) as predicated by Levene's test for homogeneity. Statistical significance in the quantified fluorescent signals for Ki67 and vWF was assessed by two‐way ANOVA with a multiple comparison post hoc test, using the analysis software GraphPad Prism 8. In all analyses, statistical significance was considered with P < .05.
3. RESULTS
3.1. Quantification of fluid accumulation at the defect site
Qualitative analysis of MR images demonstrated no substantial host response identified by T2‐weighted imaging (Figure 2A). Expectedly, the mean hyperintensity volume measured on these images peaked at the earlier time points and decreased over time (Figure 2B). Allograft peaked at 7 days postoperatively and then decreased in mean hyperintensity volume. The 4‐, 7‐, and 10‐day time points showed no significant differences among treatment groups. The high‐dose PDGF group had elevated mean volume on 7 days postoperatively compared to the other test articles, but was not statistically significant. The 21‐day time point showed significance on Welch's ANOVA (P = .028). Here, Games‐Howell post hoc testing showed that the matrix alone group had significantly higher hyperintensity volume than the low‐dose PDGF treatment group (mean difference: 17.13 mm3 ± 4.23, P = .046). Although not significant on Games‐Howell post hoc test, the allograft group typically trended higher in mean volumes relative to the test article groups.
FIGURE 2.
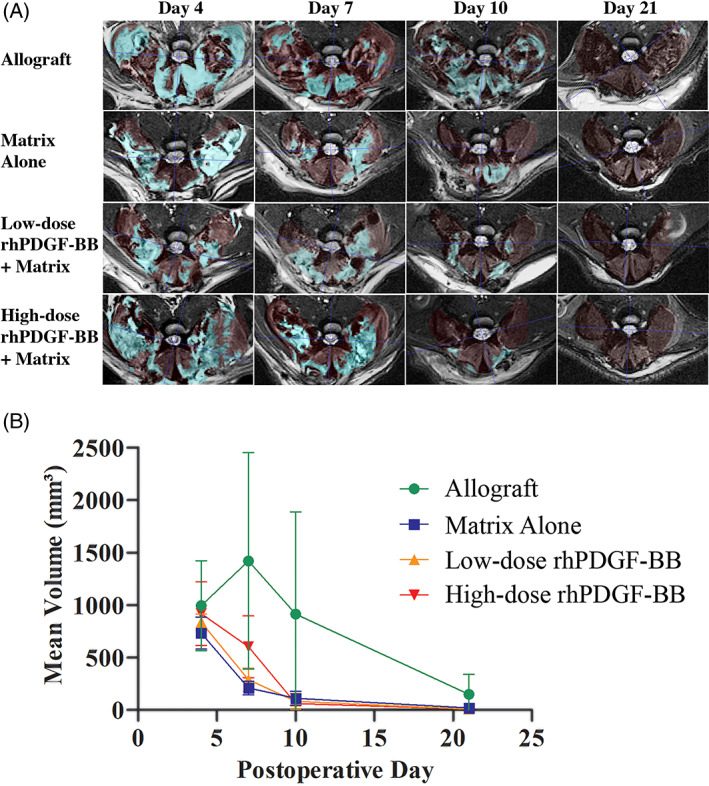
MRI analysis data with qualitative axial slices (A) and quantitative volumes of hyperintensity over time (B). (A) Representative T2‐weighted axial MRI slices of the lumbar spine at L4‐L5 disc space with overlays from ITK‐SNAP. The light red overlay indicates the muscle tissue that was selected. The aqua overlay indicates the autosegmented areas of hyperintensity, representing areas of inflammation. (B) The mean volume of hyperintense areas on T2‐weighted axial MRI slices over time. No statistically significant differences were seen among any of the groups at any of the timepoints
3.2. Serum inflammatory cytokine analysis
Four of the cytokines (GM‐CSF, EGF, GRO/KC/CINC‐1, and MIP‐2) did not meet the minimum detectable concentration (minDC) in any specimens, and these were therefore excluded from further evaluation. Twenty of the remaining 23 analytes evaluated by the Luminex xMAP technology showed no significant differences among treatment groups when segregated by time point. Notably, serum inflammatory cytokines IFN‐γ, IL‐1β, IL‐18, MCP‐1, MIP‐1α, TNF‐α were not statistically significant when segregated by time point (Figure 3). The remaining three cytokines—Leptin, IL‐6, and IL‐13—showed significant differences upon ANOVA.
FIGURE 3.
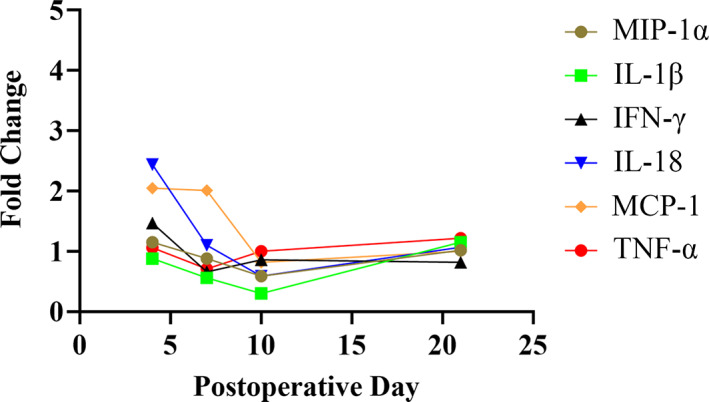
Inflammatory cytokine analysis comparing the fold‐change of the cytokines isolated in the rats with the high‐dose PDGF + matrix test implant compared to those with allograft showing no significant differences over time. IL‐18 at Day 4 and MCP‐1 at Day 7 in the rats with the implanted test articles were not statistically different. Low‐dose PDGF + matrix similarly showed no statistical differences over time
At postoperative day 7, sera concentrations of Leptin were significantly elevated in the vehicle control group relative to all other treatment groups, with post hoc P values as follows: allograft (mean difference: 3946 pg/mL, 95% CI [811, 7081]), P = .0115, low‐dose rhPDGF‐BB (mean difference: 3140 pg/mL, 95% CI [5, 6275]), P = .0496, and high‐dose rhPDGF‐BB (mean difference: 4698 pg/mL, 95% CI [1563, 7832]), P = .0029. All other time points (4, 10, and 21 days postoperatively) showed no statistically significant differences among treatment groups.
The two other cytokines that showed significance upon ANOVA did not meet the minDC in a large number of the 80 specimens. The IL‐13 analysis showed that 25 of the 80 specimens did not meet the minDC, which could have affected the statistical analysis. No significance among groups was found at the 4, 7, or 10‐day time points. At the 21‐day time point, post hoc testing demonstrated a significantly higher IL‐13 concentration in the low‐dose rhPDGF‐BB group relative to the vehicle control group (mean diff: −17, 95% CI [−33, −0.4], P = .044). Similarly, 17 of the 80 total specimens across all time points failed to meet the minDC for IL‐6. At the 10‐day time point only, post hoc testing showed that [IL‐6] was significantly lower in the high‐dose rhPDGF‐BB group relative to the vehicle control group (mean diff: 674, 95% CI [771,271], P = .024); however, only 4/5 and 2/5 of the specimens from the vehicle control and high‐dose rhPDGF‐BB treatment groups at that time point met the minDC, respectively.
3.3. Histological evaluation of cell infiltration
Representative images of H&E‐stained implant sections (Figures 4 and 5) demonstrated the β‐TCP particles appeared as empty spaces due to the decalcification process. The test materials across treatment groups were well tolerated following implantation in this model, and biological responses were similar with cellular infiltration between bone allograft (Figures 4A‐D and 5A‐D) or β‐TCP/Col matrix particles (Figures 4E‐P and 5E‐P) with new extracellular matrix deposition and neovascularization. There were no discernible differences in cellular infiltration, extracellular matrix deposition, or neovascularization among the two dose levels of rhPDGF‐BB evaluated or with matrix alone from Day 4 through Day 21 (Figures 4E‐P and 5E‐P).
FIGURE 4.
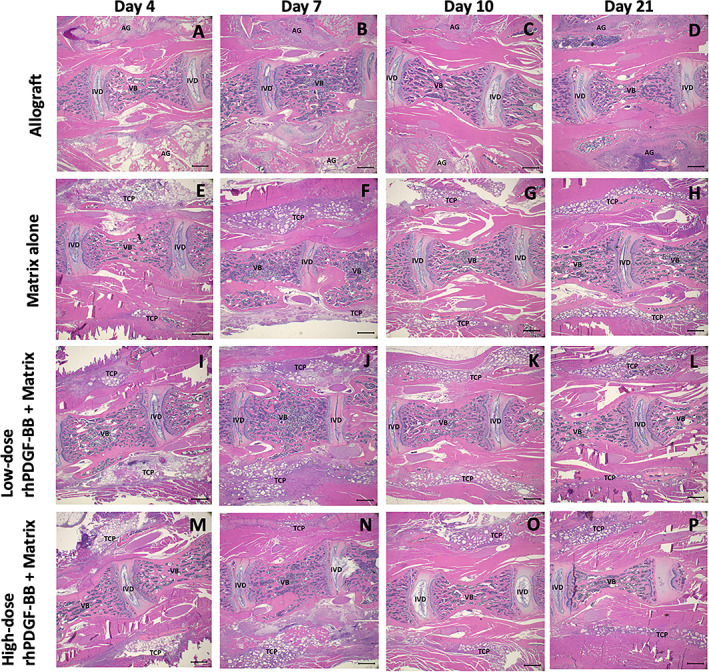
Representative H&E‐stained histology images at 1.25× magnification of each treatment group at each time point. (A‐D) Allograft. (E‐H) Matrix alone. (I‐L) Low‐dose (0.3 mg/mL) rhPDGF‐BB + matrix. (M‐P) High‐dose (3.0 mg/mL) rhPDGF‐BB + matrix. Scale bar represents 1000 μm. AG, allograft bone; IVB, intervertebral bone; IVD, intervertebral disc; TCP, β‐TCP particle
FIGURE 5.
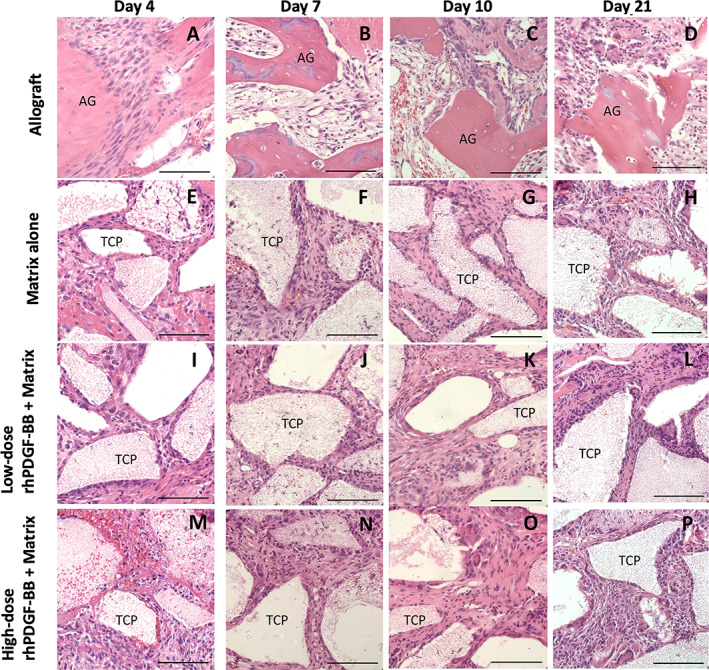
Representative H&E‐stained histology images at 40× magnification of each treatment group at each time point. (A‐D) Allograft. (E‐H) Matrix alone. (I‐L) Low‐dose (0.3 mg/mL) rhPDGF‐BB + matrix. (M‐P) High‐dose (3.0 mg/mL) rhPDGF‐BB + matrix. Scale bar represents 50 μm. AG, allograft bone; IVB, intervertebral bone; IVD, intervertebral disc; TCP, β‐TCP particle
3.4. Quantification of Ki67‐ and vWF‐positive tissue
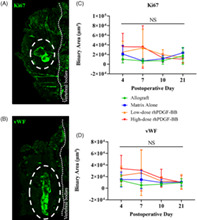
The area (binary area, μm2) of quantified fluorescent signal for Ki67 (Figure 6A) and vWF (Figure 6B) within the ROIs of analyzed tissue remained constant over time for both allograft and matrix alone. For both Ki67 and vWF, slightly higher values were recorded at 4 and 7 days postoperatively for β‐TCP/Col with low‐ and high‐dose rhPDGF‐BB, but none reached statistical significance (P > .05).
FIGURE 6.
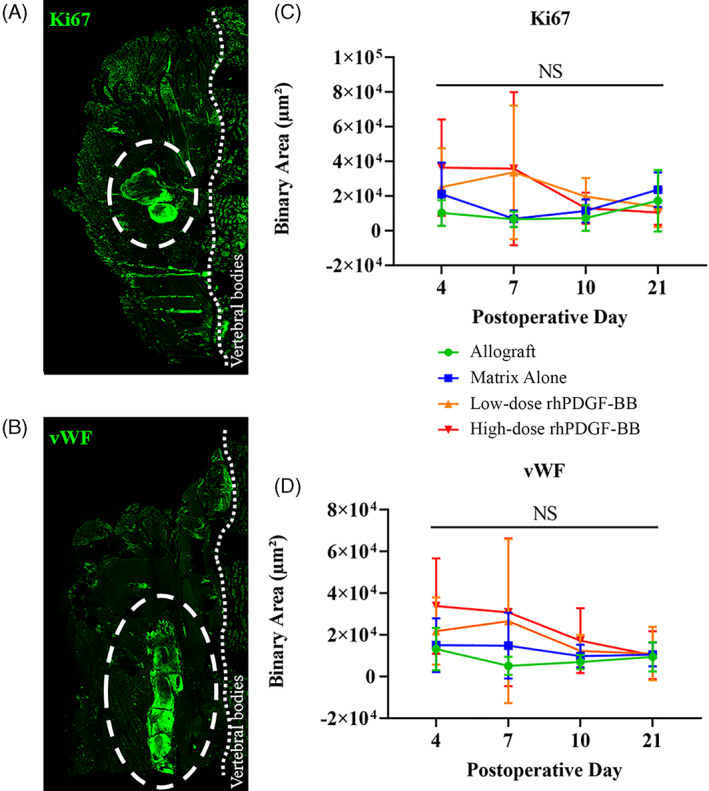
Representative immunofluorescence images of (A) Ki67‐ and (B) vWF‐stained tissue slices. Ki67 and vWF signal are shown in green. White dotted circles outline the implant region, thin dotted line indicate the vertebral bodies. Quantified Ki67 (C) and vWF (D) signal, 4, 7, 10, and 21 days postoperatively, reported as binary area (μm2). No statistically significant differences seen
4. DISCUSSION
The ideal bone graft substitute for spine fusion should simultaneously improve fusion rates and clinical outcomes while minimizing graft‐associated complications. 24 rhPDGF‐BB for therapeutic purposes has shown safety and efficacy in periodontal bone regeneration with GEM 21S (Lynch Biologics, LLC, Franklin, TN) 18 , 25 , 26 and in foot and ankle fusion with augment bone graft (Wright Medical Group, Memphis, TN). 10 , 17 , 20 , 27 , 28 , 29 Preclinical orthopedic studies, including fracture repair in animal models of impaired healing (geriatric/osteoporotic or diabetic), 13 , 14 distraction osteogenesis, 15 and spine fusion 19 have demonstrated the efficacy of rhPDGF‐BB in promoting accelerated bone repair and enhanced bone healing using concentrations of 0.3 mg/mL (low dose) to 1.0 mg/mL (high dose). Our study used a value 3× higher at 3.0 mg/mL as our high‐dose concentration in order to evaluate if there would be an inflammatory response to the graft substitute.
Given the role of PDGF within the neuroinflammatory cascade, 21 and considering the complications from case series reported with use of high‐dose rhBMP‐2, 30 , 31 , 32 the inflammatory impact of rhPDGF‐BB when administered with its carrier must be understood before considering its application for spinal fusion. While rhPDGF‐BB combined with β‐TCP demonstrated equivalent fusion rates to allograft in an ovine model, 19 no previous studies have explored the potential inflammatory effects in a preclinical model. Using a rodent arthrodesis model, we concluded that no exaggerated host inflammatory response with rhPDGF‐BB relative to allograft or vehicle controls exists.
On qualitative MR analysis with T2‐weighted axial images, animals treated with rhPDGF‐BB showed no exacerbated fluid collection at any of the post‐operative time points assessed. Quantitative analysis of signal intensity showed no statistically significant increase in animals from either low‐ or high‐dose rhPDGF‐BB treatment groups relative to the Matrix Alone or allograft control groups, and this was the case at all time points evaluated. Surprisingly, although not significant on post hoc tests, the allograft group demonstrated higher mean intensity volumes compared to the rhPDGF‐treated animals. These results contrast with previous work evaluating the host response to rhBMP‐2, which demonstrated significantly greater signal intensity (corresponding to fluid collection) in animals treated with rhBMP‐2/absorbable Type 1 collagen sponge when compared to allograft in the same animal model. 9
Of the 23 analytes quantified that reached minimal detectable concentrations, there were no significant differences in 20 of the cytokines with the exceptions being leptin, IL‐6, and IL‐13. At 7 days postoperative, sera concentrations of leptin were significantly elevated in the vehicle control group relative to all other treatment groups. Leptin is an adipocyte‐derived cytokine that links nutritional status with neuroendocrine and immune functions. 33 Initially described as an anti‐obesity hormone, leptin can affect thymic homeostasis and the secretion of pro‐inflammatory mediators such as interleukin‐1 (IL‐1) and tumor‐necrosis factor‐alpha (TNF‐α). 34 Similar to other pro‐inflammatory cytokines, leptin promotes Th1‐cell differentiation and can modulate the immune response in animal models. 35 In our study, rhPDGF‐BB did not increase leptin concentrations, but rather, the vehicle control group showed significantly elevated levels at 7 days postoperative. However, since (a) the rhPDGF‐BB groups also contained the vehicle (matrix plus sodium acetate) at the same molarity and pH; (b) the rhPDGF‐BB treatment groups did not differ from the allograft control group in sera leptin concentrations; and (c) all other time points (4, 10, and 21 days postoperative) showed no statistically significant differences among any of the treatment groups, this is unlikely to represent a true and/or clinically significant biological effect.
The differences shown in IL‐6 and IL‐13 are also unlikely to represent true biological and/or clinical effects. These two cytokines were present in rat sera at very low concentrations, and many of the specimens showed undetectable serum levels. Taking a conservative approach, those animals presenting with undetectable IL‐6 or IL‐13 serum concentrations were assigned the minDC for that particular analyte, rather than assigning a value of zero or near‐zero (which would artificially influence the results) or omitting the data point altogether. For example, at the 10‐day time point, the minDC for IL‐6 was met in only two of the five specimens from the high‐dose PDGF group. At the 21‐day time point, [IL‐13] did not meet the minDC in any of the sodium acetate control specimens, and only three of the five specimens from the high‐dose PDGF group met the minDC. Even with this conservative approach, in the few instances where the condition of P < .05 was met, the trend did not hold across multiple time points, making it even less likely to be a true effect.
For four of the analytes evaluated (GM‐CSF, EGF, GRO/KC/CINC‐1, and MIP‐2), the minDC was not met in any serum specimens, and those analytes were excluded from further evaluation. Based on this analysis, our work suggests that rhPDGF‐BB does not increase the local or systemic inflammatory response following surgical implantation in a rat posterolateral spine fusion model. The observations described here stand in contrast to previous work evaluating the host inflammatory response to rhBMP‐2, which demonstrated significant upregulation of IL‐1β, IL‐18, TNF‐α, MIP‐1α, and MCP‐1 in animals treated with rhBMP‐2/absorbable Type 1 collagen sponge in the same animal model. 9
Previous literature has described a role for endogenous PDGF in the initial response to biomaterials and wound healing. 36 PDGF‐BB is a crucial immunomodulatory chemokine and serves as a strong mitogenic and chemotactic agent that aids in recruiting cells of mesenchymal origin (osteoblasts) to sites of bone healing. 37 Thus, herein, we have assessed the level of chemotaxis and mitogenic activity, as well as of neovascularization induced by rhPDGB‐BB when delivered with our vehicle. While more Ki67‐ and vWF‐positive tissue was found at the earlier time points for both β‐TCP/Col with low‐ (0.3 mg/mL) and high‐dose (3.0 mg/mL) rhPDGF‐BB compared to allograft, none of the data were found to be statistically significant.
There are several limitations of this study that are important to acknowledge, including that outcomes noted in a small animal model should be interpreted with care when translating to the clinical setting. 38 For instance, rodent models do not always precisely replicate the human immune response. In accordance with current standards of practice, we studied only female rats and follow‐up work should be performed on males to confirm similar results. Additionally, the use of a high throughput serum cytokine assay to narrow our targets for local evaluation may miss significant changes in local expression of immunomodulatory cytokines. Specifically, the serum levels of certain cytokines may not change despite a clinically relevant local change. Furthermore, we did not utilize specific immunohistochemistry targets to evaluate for macrophage or lymphocyte infiltration. In the future, evaluating in more detail the local expression of immunomodulatory cytokines and cellular response could be the key to unveiling the characteristics of the immune response to our vehicle and rhPDGF‐BB in the context of spine surgery.
5. CONCLUSION
The results described herein demonstrate that when deployed in a preclinical setting of spine fusion, rhPDGF‐BB delivered with a β‐TCP/Col matrix exerts no exaggerated systemic or local host inflammatory response when compared to iliac crest allograft bone. Further studies are needed to validate these results in a large animal model and/or humans, and additional preclinical efficacy studies should follow to validate the use of rhPDGF‐BB combined with the β‐TCP/Col matrix to efficiently promote spine fusion.
CONFLICT OF INTEREST
Wellington K. Hsu is an active consultant for Wright Medical.
AUTHOR CONTRIBUTIONS
All the authors contributed significantly to the development of this manuscript through data analysis, manuscript preparation, and analysis and editing of final manuscript.
Supporting information
Supplemental Figure S1 Representative thresholded spine tissue slice. The two ROIs are depicted in red (ROI 1) and green (ROI 2), respectively.
ACKNOWLEDGMENT
This study was sponsored in part by Wright Medical Group, Inc.
Yamaguchi, J. T. , Weiner, J. A. , Minardi, S. , Greene, A. C. , Ellenbogen, D. J. , Hallman, M. J. , Shah, V. P. , Weisz, K. M. , Jeong, S. , Nandurkar, T. , Yun, C. , Hsu, W. K. , & Hsu, E. L. (2021). Characterizing the host response to rhPDGF‐BB in a rat spinal arthrodesis model. JOR Spine, 4(4), e1173. 10.1002/jsp2.1173
Funding information Wright Medical Group, Inc.
REFERENCES
- 1. Rajaee SS, Bae HW, Kanim LEA, Delamarter RB. Spinal fusion in the United States: analysis of trends from 1998 to 2008. Spine. 2012;37(1):67‐76. [DOI] [PubMed] [Google Scholar]
- 2. Fowler BL, Dall BE, Rowe DE. Complications associated with harvesting autogenous iliac bone graft. Am J Orthop (Belle Mead NJ). 1995;24(12):895‐903. [PubMed] [Google Scholar]
- 3. Goulet JA, Senunas LE, DeSilva GL, Greenfield ML. Autogenous iliac crest bone graft. Complications and functional assessment. Clin Orthop Relat Res. 1997;339:76‐81. [DOI] [PubMed] [Google Scholar]
- 4. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein‐2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J. 2010;10(9):e1‐e6. [DOI] [PubMed] [Google Scholar]
- 5. Pradhan BB, Bae HW, Dawson EG, Patel VV, Delamarter RB. Graft resorption with the use of bone morphogenetic protein: lessons from anterior lumbar interbody fusion using femoral ring allografts and recombinant human bone morphogenetic protein‐2. Spine. 2006;31(10):E277‐E284. [DOI] [PubMed] [Google Scholar]
- 6. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP‐2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech. 2008;21(8):557‐562. [DOI] [PubMed] [Google Scholar]
- 7. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off‐label PLIF/TLIF use of bone morphogenetic protein‐2 (BMP‐2). Spine J. 2008;8(6):1011‐1018. [DOI] [PubMed] [Google Scholar]
- 8. Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein‐2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471‐491. [DOI] [PubMed] [Google Scholar]
- 9. Hsu WK, Polavarapu M, Riaz R, et al. Characterizing the host response to rhBMP‐2 in a rat spinal arthrodesis model. Spine. 2013;38(12):E691‐E698. [DOI] [PubMed] [Google Scholar]
- 10. Hollinger JO, Hart CE, Hirsch SN, Lynch S, Friedlaender GE. Recombinant human platelet‐derived growth factor: biology and clinical applications. J Bone Joint Surg Am. 2008;90(suppl 1):48‐54. [DOI] [PubMed] [Google Scholar]
- 11. Nevins M, Camelo M, Nevins ML, Schenk RK, Lynch SE. Periodontal regeneration in humans using recombinant human platelet‐derived growth factor‐BB (rhPDGF‐BB) and allogenic bone. J Periodontol. 2003;74(9):1282‐1292. [DOI] [PubMed] [Google Scholar]
- 12. Caplan AI, Correa D. PDGF in bone formation and regeneration: new insights into a novel mechanism involving MSCs. J Orthop Res. 2011;29(12):1795‐1803. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Zube L, Breitbart EA, O'Connor JP, et al. Recombinant human platelet‐derived growth factor BB (rhPDGF‐BB) and beta‐tricalcium phosphate/collagen matrix enhance fracture healing in a diabetic rat model. J Orthop Res. 2009;27(8):1074‐1081. [DOI] [PubMed] [Google Scholar]
- 14. Hollinger JO, Onikepe AO, MacKrell J, et al. Accelerated fracture healing in the geriatric, osteoporotic rat with recombinant human platelet‐derived growth factor‐BB and an injectable beta‐tricalcium phosphate/collagen matrix. J Orthop Res. 2008;26(1):83‐90. [DOI] [PubMed] [Google Scholar]
- 15. Moore DC, Ehrlich MG, McAllister SC, et al. Recombinant human platelet‐derived growth factor‐BB augmentation of new‐bone formation in a rat model of distraction osteogenesis. J Bone Joint Surg Am. 2009;91(8):1973‐1984. [DOI] [PubMed] [Google Scholar]
- 16. DiGiovanni CW, Petricek JM. The evolution of rhPDGF‐BB in musculoskeletal repair and its role in foot and ankle fusion surgery. Foot Ankle Clin. 2010;15(4):621‐640. [DOI] [PubMed] [Google Scholar]
- 17. DiGiovanni CW, Lin SS, Baumhauer JF, et al. Recombinant human platelet‐derived growth factor‐BB and beta‐tricalcium phosphate (rhPDGF‐BB/beta‐TCP): an alternative to autogenous bone graft. J Bone Joint Surg Am. 2013;95(13):1184‐1192. [DOI] [PubMed] [Google Scholar]
- 18. Nevins M, Kao RT, McGuire MK, et al. Platelet‐derived growth factor promotes periodontal regeneration in localized osseous defects: 36‐month extension results from a randomized, controlled, double‐masked clinical trial. J Periodontol. 2013;84(4):456‐464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solchaga LA, Hee CK, Aguiar DJ, et al. Augment bone graft products compare favorably with autologous bone graft in an ovine model of lumbar interbody spine fusion. Spine. 2012;37(8):E461‐E467. [DOI] [PubMed] [Google Scholar]
- 20. Friedlaender GE, Lin S, Solchaga LA, Snel LB, Lynch SE. The role of recombinant human platelet‐derived growth factor‐BB (rhPDGF‐BB) in orthopaedic bone repair and regeneration. Curr Pharm des. 2013;19(19):3384‐3390. [DOI] [PubMed] [Google Scholar]
- 21. Yang P, Manaenko A, Xu F, et al. Role of PDGF‐D and PDGFR‐beta in neuroinflammation in experimental ICH mice model. Exp Neurol. 2016;283:157‐164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Funa K, Sasahara M. The roles of PDGF in development and during neurogenesis in the normal and diseased nervous system. J Neuroimmune Pharmacol. 2014;9(2):168‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yushkevich PA, Piven J, Hazlett HC, et al. User‐guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31(3):1116‐1128. [DOI] [PubMed] [Google Scholar]
- 24. Li G, Li P, Chen Q, Thu HE, Hussain Z. Current updates on bone grafting biomaterials and recombinant human growth factors implanted biotherapy for spinal fusion: a review of human clinical studies. Curr Drug Deliv. 2019;16(2):94‐110. [DOI] [PubMed] [Google Scholar]
- 25. Jayakumar A, Rajababu P, Rohini S, et al. Multi‐centre, randomized clinical trial on the efficacy and safety of recombinant human platelet‐derived growth factor with beta‐tricalcium phosphate in human intra‐osseous periodontal defects. J Clin Periodontol. 2011;38(2):163‐172. [DOI] [PubMed] [Google Scholar]
- 26. Nevins M, Giannobile WV, McGuire MK, et al. Platelet‐derived growth factor stimulates bone fill and rate of attachment level gain: results of a large multicenter randomized controlled trial. J Periodontol. 2005;76(12):2205‐2215. [DOI] [PubMed] [Google Scholar]
- 27. Digiovanni CW, Baumhauer J, Lin SS, et al. Prospective, randomized, multi‐center feasibility trial of rhPDGF‐BB versus autologous bone graft in a foot and ankle fusion model. Foot Ankle Int. 2011;32(4):344‐354. [DOI] [PubMed] [Google Scholar]
- 28. Daniels T, DiGiovanni C, Lau JT, Wing K, Younger A. Prospective clinical pilot trial in a single cohort group of rhPDGF in foot arthrodeses. Foot Ankle Int. 2010;31(6):473‐479. [DOI] [PubMed] [Google Scholar]
- 29. Sun H, Lu PP, Zhou PH, et al. Recombinant human platelet‐derived growth factor‐BB versus autologous bone graft in foot and ankle fusion: a systematic review and meta‐analysis. Foot Ankle Surg. 2017;23(1):32‐39. [DOI] [PubMed] [Google Scholar]
- 30. Mesfin A, Buchowski JM, Zebala LP, et al. High‐dose rhBMP‐2 for adults: major and minor complications: a study of 502 spine cases. J Bone Joint Surg Am. 2013;95(17):1546‐1553. [DOI] [PubMed] [Google Scholar]
- 31. Glassman SD, Howard J, Dimar J, Sweet A, Wilson G, Carreon L. Complications with recombinant human bone morphogenic protein‐2 in posterolateral spine fusion: a consecutive series of 1037 cases. Spine. 2011;36(22):1849‐1854. [DOI] [PubMed] [Google Scholar]
- 32. Fu R, Selph S, McDonagh M, et al. Effectiveness and harms of recombinant human bone morphogenetic protein‐2 in spine fusion: a systematic review and meta‐analysis. Ann Intern Med. 2013;158(12):890‐902. [DOI] [PubMed] [Google Scholar]
- 33. Thomas T. The complex effects of leptin on bone metabolism through multiple pathways. Curr Opin Pharmacol. 2004;4(3):295‐300. [DOI] [PubMed] [Google Scholar]
- 34. Santos CL, Bobermin LD, Souza DO, Quincozes‐Santos A. Leptin stimulates the release of pro‐inflammatory cytokines in hypothalamic astrocyte cultures from adult and aged rats. Metab Brain Dis. 2018;33(6):2059‐2063. [DOI] [PubMed] [Google Scholar]
- 35. Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med. 2012;33(1):35‐45. [DOI] [PubMed] [Google Scholar]
- 36. Boni BOO, Lamboni L, Souho T, Gauthier M, Yang G. Immunomodulation and cellular response to biomaterials: the overriding role of neutrophils in healing. Mater Horizons. 2019;6(6):1122‐1137. [Google Scholar]
- 37. Wang X, Matthews BG, Yu J, et al. PDGF modulates BMP2‐induced osteogenesis in periosteal progenitor cells. JBMR Plus. 2019;3(5):e10127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schindeler A, Mills RJ, Bobyn JD, Little DG. Preclinical models for orthopedic research and bone tissue engineering. J Orthop Res. 2018;36(3):832‐840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1 Representative thresholded spine tissue slice. The two ROIs are depicted in red (ROI 1) and green (ROI 2), respectively.


