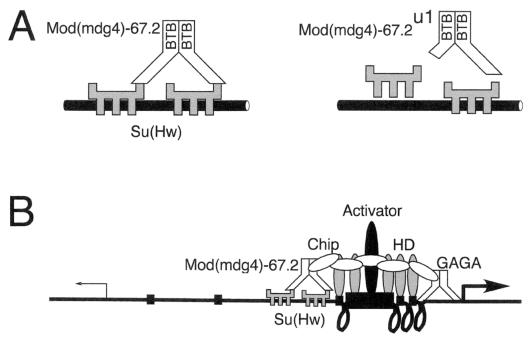
FIG. 6.
Proposed model for the role of Mod(mdg4)-67.2 protein in insulation. (A) To explain why truncated Mod(mdg4)-67.2 proteins have dominant-negative (antimorphic) effects on insulator activity, we propose, as shown on the left, that a multimer of Mod(mdg4)-67.2 interacts with more than one DNA-bound molecule of Su(Hw) to form a stable insulator complex. As shown on the right, truncated Mod(mdg4)-67.2u1 or Mod(mdg4)-67.2T6 protein would destabilize the complex, leading to reduced interaction between Mod(mdg4)-67.2 and Su(Hw) and/or reduced binding of Su(Hw) to DNA. (B) To explain how the Mod(mdg4)-67.2 protein contributes to the insulator activity of Su(Hw), we propose, as depicted on the left, that it traps facilitators such as Chip and blocks the previously postulated Chip-assisted spread of homeodomain protein (HD) binding between the enhancer and promoter (12, 55). As depicted on the right, this spread could create a series of loops that brings the enhancer and promoter closer together or could aid the binding of a surrogate activator near the promoter. Like the Mod(mdg4) proteins, GAGA factor is a group of BTB/POZ-containing trxG proteins that can also both aid activation and help insulate (44, 45). GAGA factor binds proximal to several promoters and potentiates activation of the engrailed promoter by a distal enhancer (46). We speculate, as shown with the promoter on the right, that when BTB/POZ proteins such as GAGA bind just upstream of a promoter, they anchor activators close to the promoter and thereby aid activation.