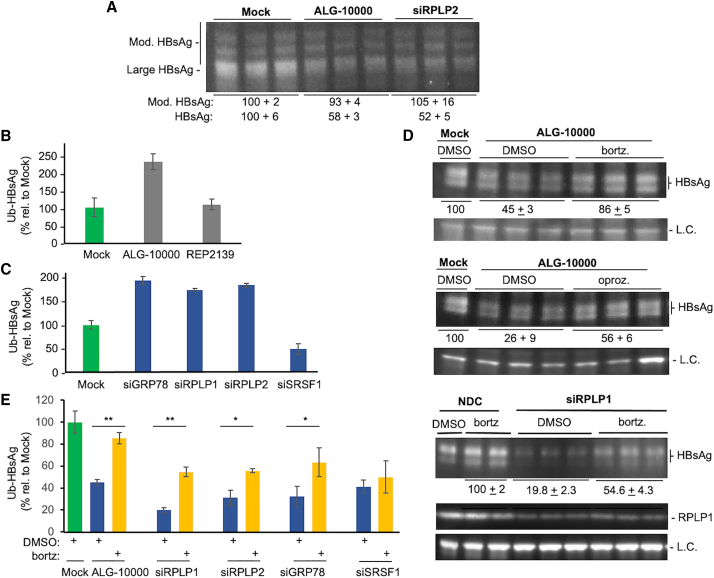
Figure 7.
STOPS reduction of HBsAg involves ubiquitination of the HBsAg and proteasome degradation
(A) HBsAg has post-translational modifications. The HBsAg was from HepG2.2.15 cells treated with 8 nM ALG-10000 or 10 nM siRPLP2 for 76 h. The most prominent band is the molecular mass expected of the large HBsAg. Additional higher-molecular-weight bands may be ubiquitinated forms of the HBsAg. (B) Intracellular HBsAg in HepG2.2.15 cells treated with ALG-10000 increased the amount of ubiquitin. The ubiquitinated HBsAg assayed was from cells treated for 76 h with 8 nM ALG-10000. (C) Knockdown of GRP78, RPLP1, and RPLP2 can increase HBsAg ubiquitination. All siRNAs were transfected into HepG2.2.15 cells at a final concentration of 10 nM. The amount of ubiquitinated HBsAg was normalized to the amount of HBsAg present in the cell lysate. (D) Proteasome inhibitors can partially reverse ALG-10000-mediated reduction of HBsAg and RPLP1 levels. The images of the western blot show the large HBsAg in HepG2.2.15 transfected with 10 nM ALG-10000 and treated with the proteasome inhibitor bortezomib (25 nM), or oprozomib (100 nM), or DMSO, the vehicle used to solubilize the proteasome inhibitors. The cells were treated for 24 h before their lysis for western blot analysis. The LC is the protein GAPDH. siRPLP1 was transfected at 10 nM and the cells treated with bortezomib are as described in (A). (E) The amount of ubiquitinated HBsAg present in HepG2.2.15 cells after treatment with ALG-10000 or knockdown of GRP78, RPLP1, and RPLP2 is increased by treatment with the proteasome inhibitor bortezomib. ∗p < 0.05, ∗∗p < 0.01. In the graphs in panels D, C, and E, each error bar represents one standard deviation of uncertainty.