Figure 2.
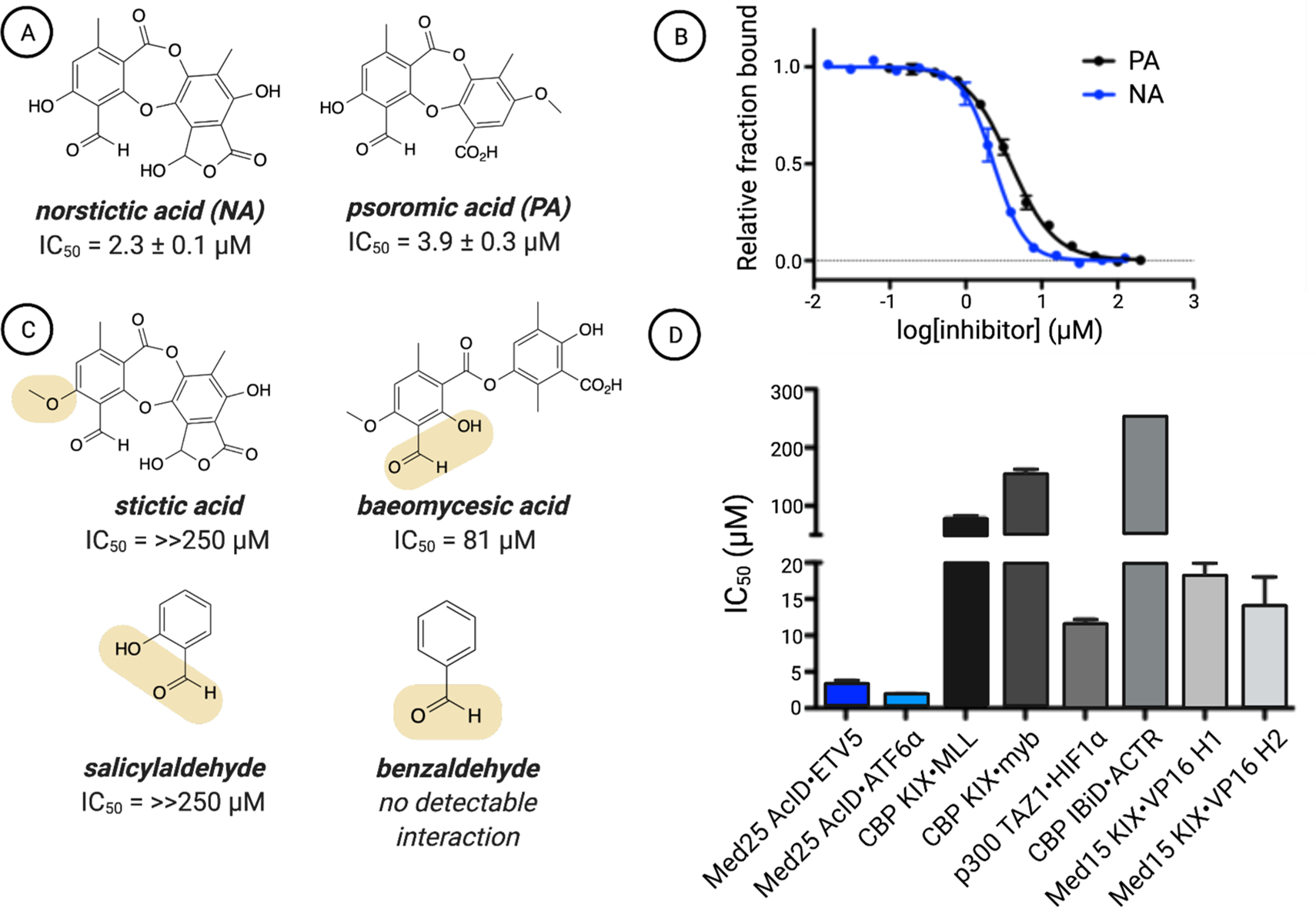
(A) Chemical structures of the top two hits emerging from the screen of Med25 AcID fl-VP16(465–490) along with (B) their apparent IC50 values, which were determined through titrations of either NA or PA against Med25 AcID fl-VP16(465–490) performed in triplicate with the indicated error (SDOM). Full experimental details are reported in the Supporting Information. (C) Assessment of related structures shows that the orthophenoxyaldehyde moiety is important but not sufficient for inhibitory activity. IC50 values were determined via competition fluorescence polarization against Med25 VP16(465–490). Of these molecules, covalent adducts with Med25 were observed only for baeomycesic acid, indicating that the orthophenoxyl group is integral to stable imine formation. (D) Inhibition of related PPI networks by NA. Apparent IC50 values were measured via fluorescence polarization against a suite of coactivator domains (CBP KIX, p300 TAZ1, CBP IBiD, Med15 KIX) bound to fluorescein-tagged activators. The values are averages of three independent experiments with the indicated error (SDOM). No error bars are shown for the IC50 against IBiD ACTR because the IC50 was greater than the highest concentration of NA tested (250 μM), and thus, we can accurately report the IC50 only as >250 μM. Full details are reported in the Supporting Information.
