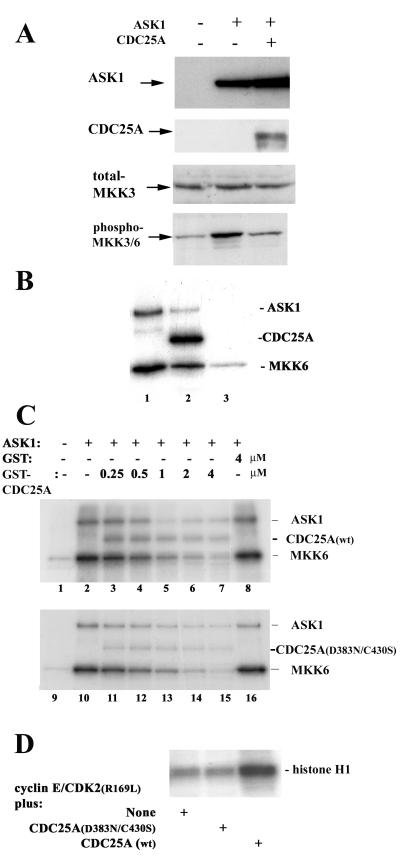
FIG. 3.
CDC25A inhibits ASK1 in a manner independent of its phosphatase activity. (A) Decreased ASK1-dependent MKK phosphorylation by cotransfection with CDC25A. COS-7 cells were transfected with pcDNA3 vectors encoding the proteins shown in the top panel. Cell extracts were prepared at 48 h after transfection and analyzed by immunoblotting with antibodies for ASK1, CDC25A, MKK3, and phosphorylated active MKK3/6. (B) CDC25A is both an inhibitor and a substrate of ASK1. ASK1 was immunoprecipitated from Sf9 cells infected with a human ASK1-encoding recombinant baculovirus and incubated at 30°C for 30 min with purified GST (lane 1) or GST-CDC25A (lane 2) in the presence of [γ-32P]ATP and purified GST-MKK6 (kinase-inactive KM mutant form). Immunoprecipitates with normal IgG were used as a negative control (lane 3). Phosphorylation of the proteins was analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) and autoradiography. (C) Inhibition of ASK1 by CDC25A and CDC25A(D383N/C430S). Extracts of Sf9 cells infected with the ASK1-encoding baculovirus were preincubated for 30 min at 30°C with purified GST-CDC25A, GST-CDC25A(D383N/C430S), or GST at the indicated concentration, before the addition of [γ-32P]ATP and GST- MKK6(KM). After incubation for 30 more min, phosphorylation of the proteins was analyzed by SDS-PAGE and autoradiography. Extracts of uninfected Sf9 cells were used as negative controls (lanes 1 and 9). wt, wild type. (D) The CDC25A(D383N/C430S) double mutant is phosphatase inactive. The activity of CDC25A was assessed by its ability to activate cyclin E-CDK2 in vitro. Sf9 cells were coinfected with baculoviruses for the expression of human cyclin E and CDK2(R169L), a mutant CDK2 known to accumulate inhibitory phosphorylation at Tyr 15. Cyclin E-CDK2 complexes were immunoprecipitated with anti-CDK2 antibody and incubated at 30°C for 30 min with GST-CDC25A(D383N/C430S) or wild-type GST-CDC25A. CDK2 kinase activity then was measured with histone H1 as a substrate, and phosphorylation of histone H1 was analyzed by SDS-PAGE and autoradiography.