Abstract
Introduction
Microsatellite instability (MSI) testing and tumor mutational burden (TMB) are genomic biomarkers used to identify patients who are likely to benefit from immune checkpoint inhibitors. Pembrolizumab was recently approved by the Food and Drug Administration for use in TMB-high (TMB-H) tumors, regardless of histology, based on KEYNOTE-158. The primary objective of this retrospective study was real-world applicability and use of immunotherapy in TMB/MSI-high patients to lend credence to and refine this biomarker.
Methods
Charts of patients with advanced solid tumors who had MSI/TMB status determined by next generation sequencing (NGS) (FoundationOne CDx) were reviewed. Demographics, diagnosis, treatment history, and overall response rate (ORR) were abstracted. Progression-free survival (PFS) was determined from Kaplan–Meier curves. PFS1 (chemotherapy PFS) and PFS2 (immunotherapy PFS) were determined for patients who received immunotherapy after progressing on chemotherapy. The median PFS2/PFS1 ratio was recorded.
Results
MSI-high or TMB-H [≥20 mutations per megabase (mut/MB)] was detected in 157 adults with a total of 27 distinct tumor histologies. Median turnaround time for NGS was 73 days. ORR for most recent chemotherapy was 34.4%. ORR for immunotherapy was 55.9%. Median PFS for patients who received chemotherapy versus immunotherapy was 6.75 months (95% confidence interval, 3.9-10.9 months) and 24.2 months (95% confidence interval, 9.6 months to not reached), respectively (P = 0.042). Median PFS2/PFS1 ratio was 4.7 in favor of immunotherapy.
Conclusion
This real-world study reinforces the use of TMB as a predictive biomarker. Barriers exist to the timely implementation of NGS-based biomarkers and more data are needed to raise awareness about the clinical utility of TMB. Clinicians should consider treating TMB-H patients with immunotherapy regardless of their histology.
Key words: microsatellite instability, tumor mutational burden, precision medicine, immune checkpoint inhibitors, biomarker
Highlights
-
•
This retrospective study examined the real-world use of immune checkpoint inhibitors (ICIs) in TMB/MSI-high patients with a diverse set of cancer types.
-
•
TMB is an emerging tumor-agnostic biomarker for response to treatment with ICIs that may expand personalized cancer care.
-
•
ICIs remain underutilized as a first-line therapy for TMB/MSI-H patients without specific histologic approval for ICIs.
-
•
The PFS2 to PFS1 ratio was 4.7, favoring immunotherapy over chemotherapy even as a second-line therapy.
-
•
Our study reinforces the real-world evidence that TMB is a valid surrogate marker for MSI and can predict response to ICIs.
Introduction
By activating the immune system, immune checkpoint inhibitors (ICIs) unleash a powerful antitumor effect. Unfortunately, beyond the known histologies sensitive to immunotherapy, such as melanoma and non-small-cell lung cancer, few predictive biomarkers exist.1, 2, 3, 4 Many studies have looked at prognostic factors related to benefit from immunotherapy, such as neutrophil-lymphocyte ratio and programmed death-ligand 1 (PD-L1) positivity.5 Despite hope of PD-L1 as an early predictive biomarker, it has shown limited utility in predicting response to ICIs in many clinical settings.1, 2, 3, 4
Microsatellite instability (MSI) has emerged as a ‘predictive’ biomarker of benefit from immunotherapy. Le et al.6 showed that colorectal cancer patients with mismatch repair defects [MSI-high (MSI-H)] were responsive to immunotherapy. The overall response rate (ORR) for mismatch repair-deficient patients was 40% compared with 0% for mismatch repair-proficient patients. Based on the results of this study, as well as numerous others, the Food and Drug Administration (FDA) approved pembrolizumab for the treatment of MSI-H solid tumors in 2017.7
More recently, tumor mutational burden (TMB) has surfaced as a quantitative genomic biomarker of response to ICIs in the quest for additional biomarkers beyond PD-L1 staining and MSI.8, 9, 10, 11 In Goodman et al,4 the response rate to anti-programmed cell death protein 1 (PD-1)/anti-PD-L1 therapy was 58% and 20% for TMB-high (TMB-H) and TMB-low patients, respectively, indicating an association between TMB and improved response to immunotherapy. In the pivotal KEYNOTE-158 study, the ORR was 29% in TMB-H tumors compared with 6% in non-TMB-H tumors.12 These favorable results to immunotherapy in a diverse set of tumor types led to the second tissue-agnostic FDA approval for pembrolizumab.
Several important limitations of the KEYNOTE-158 study generated debate, leading to questions on TMB as a biomarker.13,14 The first limitation was the larger generalizability of this study given the relatively small number of TMB-H patients enrolled (N = 102). Additionally, a corresponding significant progression-free survival (PFS) or overall survival (OS) advantage was not observed despite the clear difference in ORR. Third, a noticeable overlap existed between MSI-H and TMB-H tumors in the study. Finally, the threshold for high TMB of ≥10 mutations per megabase (mut/Mb) remains controversial, and may be dependent on histology.8,15,16
One proposed TMB cut-off for non-small-cell lung cancer (NSCLC) is defined by ≥10 mut/Mb, and is associated with increased response rates and improved PFS in patients with metastatic NSCLC when treated with a combination of immunotherapies.17 Earlier work to define TMB as a biomarker, however, used a cut-off of >20 mut/Mb.4,18,19 Additionally, in the KEYNOTE-158 trial, response rates in the 10-13 mut/Mb group were significantly lower than those patients with >13 mut/Mb, highlighting that the chosen cut-point may not be optimal to separate responders from non-responders.14 Higher TMB cut-offs will have greater specificity and less sensitivity.
Recently, several authors have raised questions about the clinical utility of TMB, demonstrating that high TMB may not predict checkpoint response across all tumor types20 and raising the possibility that this is an imperfect biomarker21 that needs refinement.22 One proposed limitation of TMB-H as a biomarker for response to ICIs is in how TMB is defined, as the total number of mutations present in a tumor sample.21 It is assumed that the more mutations, and thus the higher the TMB, the more likely a patient’s immune system will be activated to target their tumor’s neoantigens.23 Using TMB as a biomarker, in isolation, does not take into account that only a small number of mutations may result in immune recognizable neoantigens or that a specific tumor microenvironment, such as one that hinders the trafficking of T cells, may limit the patient’s anti-neoantigen immune response.21 There is no perfect biomarker, however, and further confirmatory evidence is urgently needed to truly refine TMB into a clinically robust biomarker. Given these unanswered questions and the paucity of real-world data about TMB use as a predictive biomarker of response to ICIs across tumor types, we conducted a retrospective study of MSI-H and TMB-H (using a cut-off of >20 mut/Mb, according to FoundationMedicine methodology) in patients with multiple solid tumors who received immunotherapy. In addition, an exploratory objective of this study was clinical application of these biomarkers by clinicians and their potential real-world utility.
Materials and methods
Patient population
We reviewed the electronic medical records of patients with solid tumors who received comprehensive genomic profiling at our academic medical center. This retrospective review was conducted according to Institutional Review Board guidelines as part of an approved protocol (IRB Study #Pro2018002434). Adult patients who had TMB-H and/or MSI-H tumors were identified. MSI and TMB status were determined by using next generation sequencing (NGS) (FoundationOne CDx, a CLIA-approved laboratory) on a 324-gene panel. TMB-H was designated as per Foundation Medicine as ≥ 20 mut/Mb. This higher mutation rate was chosen because this study was retrospective and patients were treated before the results of KEYNOTE-158 were available; thus, a TMB-H cut-off of 20 mut/Mb was standard at the time these patients were treated.
Date of tumor specimen collection, date specimen was received by Foundation Medicine, and date the report was received by our cancer center were abstracted from FoundationOne reports. These dates were used to calculate the median time from specimen collection to receipt of NGS and the median time from specimen receipt to finalized report.
Data analysis
Eligible patient charts were reviewed for demographics, tumor histology, date of diagnosis, stage at diagnosis, treatment history, and best treatment response as based upon imaging or clinical observation. Patients were classified as immunotherapy-treated or chemotherapy-treated. PFS was measured, based on assessment by the treating physician, from start of treatment to date of progression and was calculated independently for both chemotherapy and immunotherapy. These values were compared using chi-square tests with significance thresholds of 0.05. PFS was determined from Kaplan–Meier curves. PFS 1 (PFS on chemotherapy) and PFS 2 (PFS on subsequent immunotherapy) were determined for patients who received immunotherapy after progressing on chemotherapy. Similar to methodology from Schwaederle et al,24 PFS2/PFS1 ratio was recorded. Number of patients with a PFS ratio ≥1.3 was recorded. ORR was calculated using complete and partial responses. Disease control rate was defined as stable disease for >6 months, complete response, or partial response.
Additionally, the immunotherapy-treated group was subdivided into MSI-H and TMB-H. The PFS for these subgroups were estimated on Kaplan–Meier curves and compared using chi-square tests with significance thresholds of 0.05. The ORR was also calculated.
Results
Patient characteristics
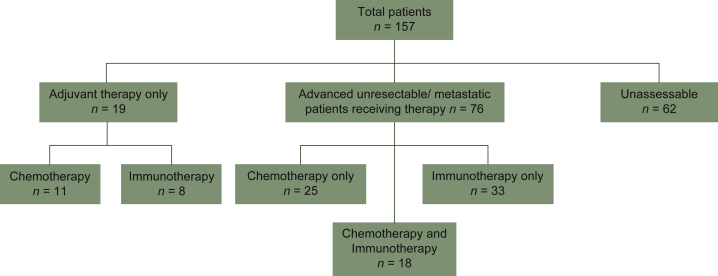
MSI-H or TMB-H results, as determined by FoundationOne CDx, were available for 157 adult patients with solid tumor malignancies. During this time period, there were 2265 comprehensive genomic profiles sent at the institution for a TMB-H and/or MSI-H rate of 6.9%. Our patient cohort included those patients who were MSI-H, TMB-H, or both from our institution. Of these 157 patients, 35 (22.3%) were MSI-H, 149 (94.9%) were TMB-H, and 27 (17.2%) were both MSI-H and TMB-H. The male to female ratio was 48% to 52% and the median age at cancer diagnosis was 66 years. There were 27 distinct histologies represented, with the most common being melanoma (56/157, 35.7%), endometrial adenocarcinoma (24/157, 15.3%), lung adenocarcinoma (20/157, 12.7%), and colon adenocarcinoma (8/157, 5.1%) (Table 1). There were 33 patients with stage IV disease at time of diagnosis and there were 72 patients who progressed to stage IV after their initial staging for a total of 105 patients with metastatic disease. Of these, 76 were assessable and received therapy for their metastatic disease (Figure 1). Of the patients in this retrospective study, 95 were alive as of data cut-off. PD-L1 staining was not available for a large proportion of patients and was therefore excluded from analysis in this study.
Table 1.
Patient demographics and baseline characteristics
| Patients (N = 157) | |
|---|---|
| Sex, n (%) | |
| Female | 81 (52) |
| Male | 76 (48) |
| Age at diagnosis (years) | |
| Median (range) | 66 (18-91) |
| Tumor type, n (%) | |
| Melanoma | 56 (35.7) |
| Endometrial | 24 (15.3) |
| Lung adenocarcinoma | 20 (12.7) |
| Colon | 8 (5.1) |
| Skin squamous cell | 7 (4.5) |
| Lung squamous cell | 7 (4.5) |
| Urothelial | 5 (3.2) |
| Unknown primary squamous cell | 4 (2.5) |
| Cervical squamous cell | 2 (1.3) |
| Uterine carcinosarcoma | 2 (1.3) |
| Small-cell lung cancer | 2 (1.3) |
| Large-cell neuroendocrine | 2 (1.3) |
| Prostate | 2 (1.3) |
| Unknown primary | 2 (1.3) |
| Ovarian | 2 (1.3) |
| Gastric | 2 (1.3) |
| Uterine leiomyosarcoma | 1 (0.6) |
| Breast | 1 (0.6) |
| Glioblastoma | 1 (0.6) |
| Clear cell endometrial | 1 (0.6) |
| Head and neck | 1 (0.6) |
| Kidney sarcomatoid | 1 (0.6) |
| Merkel cell | 1 (0.6) |
| Small intestine | 1 (0.6) |
| Pancreatobiliary | 1 (0.6) |
| Soft tissue sarcoma | 1 (0.6) |
| Adrenal gland | 1 (0.6) |
| Treatmenta, n (%) | |
| Prior radiotherapy | 56 (35.7) |
| Adjuvant therapy | 34 (21.7) |
| Immunotherapy | 59 (37.6) |
| Chemotherapy | 54 (34.4) |
| Genomic status,an (%) | |
| MSI-H | 35 (22.3) |
| TMB-H | 149 (94.9) |
| TMB-H and MSI-H | 27 (17.2) |
MSI-H, microsatellite instability-high; TMB-H, tumor mutational burden-high.
Patients may fall into more than one category.
Figure 1.
Diagram representing our patient population available for analysis.
Patients who received both chemotherapy and immunotherapy in the metastatic/unresectable setting (N = 18) are noted. A total of 15 out of these 18 patients were used to calculate the progression-free survival ratio (PFS2/PFS1) as they received chemotherapy before immunotherapy.
Foundation medicine reporting timetables
All 157 patients had a tumor specimen sent to Foundation Medicine for genomic analysis. The median time from specimen collection to arrival at Foundation Medicine for analysis was 60 days (6-2176 days). The median time from Foundation Medicine’s receipt of the specimen to our retrieval of the finalized report was 13 days (1-57 days). Therefore, the total time, from initiation of the process, it took for physicians to receive a completed genomic analysis was a median of 73 days.
Therapy
Adjuvant therapy was received by 34 patients, of whom 26 received chemotherapy and 8 received immunotherapy. All eight patients who received adjuvant immunotherapy had melanoma. None of the 8 patients with adjuvant melanoma had recurrent disease, and 13 out of 26 patients who received adjuvant chemotherapy recurred. Out of the 26 adjuvant chemotherapy patients, 2 had received prior neoadjuvant chemotherapy; therefore 19 patients received adjuvant therapy only. No histology was associated with recurrent disease.
Overall, 59 (59/157, 37.6%) patients received systemic immunotherapy, 54 (54/157, 21.7%) received chemotherapy, and 18 (18/157, 11.5%) received both immunotherapy and chemotherapy at different timepoints. Of these patients, 36 received chemotherapy only and 41 received immunotherapy only. A total of 62 patients did not receive any systemic therapy as they underwent a curative surgery, chose palliative care, or their most recent records were not available. In the metastatic or advanced unresectable setting, immunotherapy alone was given to 33 patients and chemotherapy alone was given to 25 patients (Figure 1). The most common immunotherapy regimen was the anti-PD-1 blocking antibody, pembrolizumab, which was used as monotherapy in 30 patients. Sixteen patients received immunotherapy on a clinical trial protocol. No patients received simultaneous chemotherapy and immunotherapy as part of their last treatment regimen.
Responses
Overall: chemotherapy versus immunotherapy
For patients who received chemotherapy alone, the ORR of their most recent regimen was 34.4%. For all patients who received immunotherapy, as first line or later, the ORR was 55.9%. For those who received immunotherapy as a first-line therapy and did not receive any chemotherapy, the ORR was 63.4%.
MSI-H versus TMB-H
The ORR for MSI-H and TMB-H patients who only received immunotherapy was 50.0% and 64.1%, respectively. Those who were both TMB-H and MSI-H had an ORR of 50% and a disease control rate of 75%. Four patients (12.5%) achieved a complete response to chemotherapy and nine (22%) to immunotherapy.
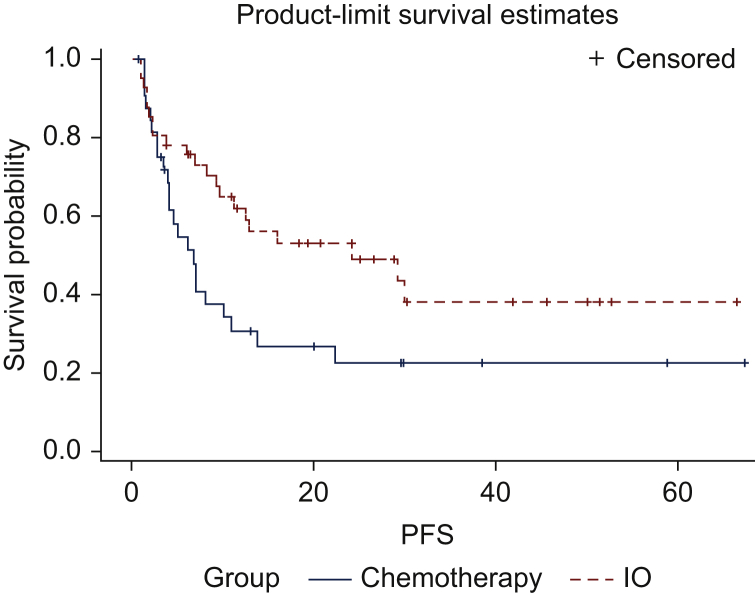
Among MSI-H and TMB-H patients, median PFS for patients who received chemotherapy alone versus immunotherapy alone was 6.75 months [95% confidence interval (95% CI), 3.9-10.9 months] and 24.2 months (95% CI, 9.6 months to not reached), respectively, with a P value of 0.042 (Figure 2).
Figure 2.
Kaplan–Meier analysis of progression-free survival as assessed by treating physician.
The solid blue line represents microsatellite instability-high (MSI-H) and/or tumor mutational burden-high (TMB-H) patients treated with chemotherapy alone and the dashed red line represents MSI-H and/or TMB-H patients treated with immunotherapy alone. The hatch marks represent censored patients. Median PFS for chemotherapy was 6.75 months and for immunotherapy was 24.2 months (P = 0.042).
IO, immunotherapy; PFS, progression-free survival.
PFS2 versus PFS1
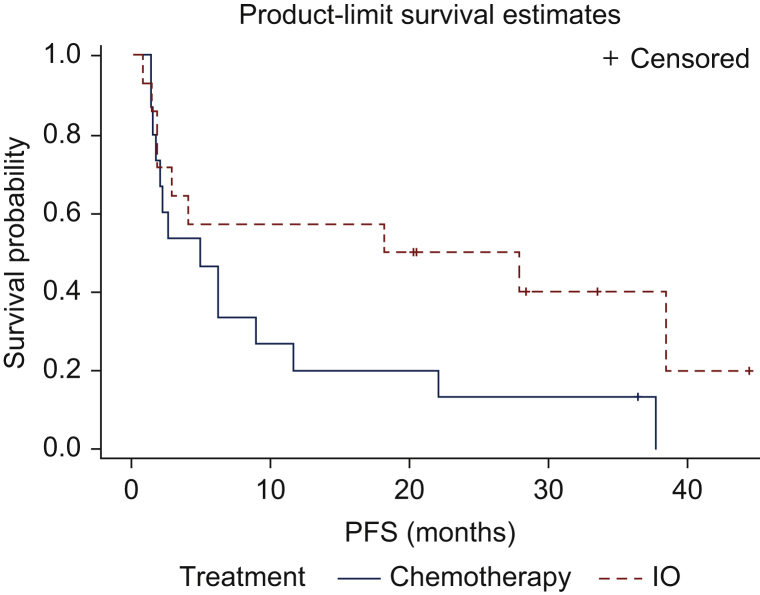
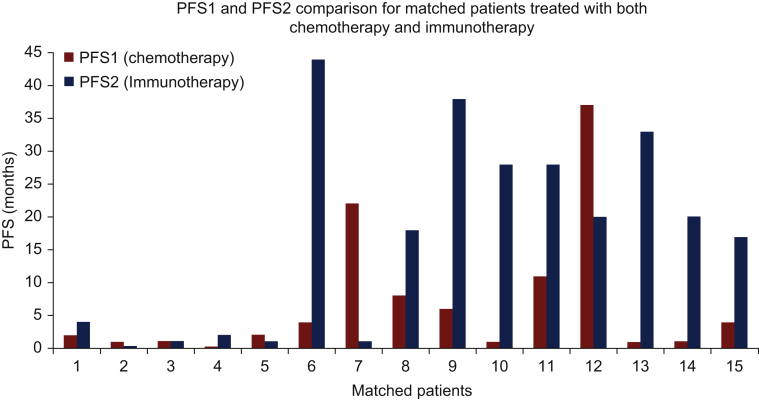
There were 18 patients who received chemotherapy and immunotherapy at different timepoints in their history. A total of 15 of these patients received immunotherapy following progression on chemotherapy. Only 1 of these 15 patients had melanoma. The median PFS for chemotherapy in this cohort of 15 patients was 4.9 months and for immunotherapy was 23 months (Figure 3). The median PFS2 to PFS1 ratio was 4.7 (Figure 4).
Figure 3.
Progression-free survival in patients who, received immunotherapy (red line, PFS2) following progression on chemotherapy (blue line, PFS1).
Median progression-free survival (PFS) for last line of chemotherapy was 4.9 months (PFS1) and for subsequent immunotherapy was 23 months (PFS2). The median PFS2 to PFS1 ratio was 4.7. Since immunotherapy follows progression after chemotherapy, these survival curves should not be directly compared with each other.
IO, immunotherapy.
Figure 4.
Bar graph progression-free survival comparisons for the patients who received both chemotherapy (red bars, PFS1) and immunotherapy (blue bars, PFS2).
PFS2/PFS1 ratio >1.3 was observed in 10/15 (67%) patients.
PFS, progression-free survival.
Discussion
MSI-H and TMB-H are rare genomic events in the overall cancer population. Only 6.9% of patients with NGS at our institution were either MSI-H or TMB-H. Given the recent tumor-agnostic approvals for immunotherapy, these biomarkers are becoming more relevant in the treatment decision-making process, especially for patients with tumor types that do not yet have immunotherapy approval. Our retrospective study aimed to confirm the clinical benefit of immunotherapy in MSI-H or TMB-H tumors in clinical practice. These results are important in illustrating the effectiveness of immunotherapy in this subset of patients and highlight the ‘underutilization’ of these biomarkers in the real-world setting.
The response rate of MSI-H and/or TMB-H patients to immunotherapy is consistent with prior reports at an ∼50% response rate. In our study, as in Carbone et al,25 patients had a lower response rate to chemotherapy, suggesting that immunotherapy is the preferred agent to use in this population. This was confirmed in the subset of this population who received both immunotherapy and chemotherapy. In those patients who received both immunotherapy and chemotherapy, the median PFS ratio was 4.7 with a majority of patients having outsized benefit from ICI. This suggests that immunotherapy is more effective than chemotherapy, even when used in the second line or later. This is based on data predominantly excluding melanoma patients who have a known lack of response to chemotherapy. Prior studies have shown that grade 3 or 4 adverse events occurred in 51% of those treated with chemotherapy, but only occurred in 18% of patients treated with immunotherapy.7 Additionally, when these adverse events occurred in immunotherapy patients, the toxicity was more manageable, especially with the use of corticosteroids.26
As immunotherapy shows a greater clinical benefit than chemotherapy in this group of patients, it is important to know which genetic biomarker confers the best response. An important observation from this study is that the response rates of TMB-H versus MSI-H tumors to treatment with immunotherapy were not significantly different. This suggests that both TMB and MSI are good predictors of response and that TMB is a good surrogate independent of MSI status. These findings confirm the results found in Goodman et al.,18 which reported a significantly longer median PFS (26.8 versus 4.3 months, P = 0.0173) in TMB-H tumors, regardless of MSI status, in a larger (148 803 patients) but similarly heterogeneous group of cancer subtypes. The prognostic value of TMB-H was further confirmed by a recent meta-analysis from Galvano et al.27 in which both PFS (HR 0.69, 95% CI 0.61-0.79) and OS (HR 0.67, 95%, CI 0.59-0.77) were significantly prolonged in TMB-H patients treated with first-line immunotherapy. Although the vast majority of MSI-H patients were TMB-H as well, the majority of TMB-H patients were not MSI-H, showcasing the additive utility of this biomarker to MSI-H testing. Our dataset suggests that TMB-H is significantly more common than MSI-H and has a similar predictive value for response to immunotherapy, suggesting that it has more clinical applicability.
Despite the overall tissue-agnostic nature of TMB and MSI as biomarkers, there appears to be an associated dependency on histology as well. For example, a leiomyosarcoma patient was observed to have a TMB of 26 and a PD-L1 staining of >90%, but derived no benefit from PD-1-directed therapy. Anecdotally, this has been observed in leiomyosarcoma previously and suggests that at least in this histology, traditional immunotherapy biomarkers are not useful.28,29 Furthermore, in other histologies, such as Kaposi sarcoma18 and Merkel cell carcinoma, response to ICIs has been shown in the absence of TMB-H.21 These findings of responsiveness or nonresponsiveness to ICI independent of TMB status highlight that other factors affecting ICI response, including but not limited to universal mutational signatures and patient specific genomic alterations, and the level of heterogeneity of a patient’s T-cell receptors, may play a larger additive role than TMB in determining response to ICIs in certain histologies.21 Further study is warranted. Furthermore, although TMB-H and MSI-H are strongly correlated and both associated with increased neoantigens,30 there may be varying underlying causes that result in TMB-H versus MSI-H. The interdependence and heterogeneity between these two biomarkers remain under investigation.31,32
Our study is limited by heterogeneity of tumor types and staging and variety of immunotherapy treatments. The purpose of TMB, however, is to be tumor agnostic and work despite tumor heterogeneity as an agnostic biomarker and is not specific to a particular immunotherapy. Additionally, we acknowledge that the majority of patients were represented by three histologies (melanoma, NSCLC, and endometrial cancer). This may not materially skew key results such as PFS2/PFS1 ratio, as at least the melanoma patients were largely excluded from that analysis since they do not generally receive chemotherapy. Our study spanned several years including predating the routine combination of chemotherapy and immunotherapy in NSCLC and is therefore an opportunity to evaluate the sequential utility of these agents rather than as a combination.
One interesting result is the ‘underutilization’ of immunotherapy for patients who were either MSI-H or TMB-H. Out of 108 patients, only 45 were treated with immunotherapy. We posit that physicians may have been unaware of the positive correlation between MSI, TMB, and response to immunotherapy, especially those treated before the wide reporting of this interrelationship. We expect this to change with the FDA acceptance of TMB-H as a biomarker. We observed that front-line immunotherapy was utilized almost exclusively in diseases where it is FDA approved in this setting, regardless of a biomarker. Our data outline several reasons for this. One is the ‘significant’ delay between the time the specimen is collected and NGS is ordered or a sample sent, suggesting that physicians may not be ordering NGS in a timely manner at diagnosis or that there are significant processing and shipping delays. Additionally, the turnaround time for NGS results to arrive ranged from 2 to 4 weeks and may be beyond the time needed to make a frontline treatment decision. We must also factor into these delayed results the time it takes for a clinician to view the report and discuss their implications with the patient.
Finally, we reflect on the unanswered questions from KEYNOTE-158. Regarding generalizability, our data add to the growing body of literature affirming KEYNOTE-158 under real-world conditions. While our study did not include TMB-low patients as a control, anecdotally, patients who are MSI-H or TMB-H have a long PFS. By setting a higher TMB threshold (20 mut/Mb), the results appear to be even more pronounced. It is now established that checkpoint inhibitors have a role in TMB >10 and it is clear from our study and others that a TMB >20 has more significant benefit. The area between these cut-offs is likely a fluid zone of increasing predictive value. Future research should focus on the clinical significance between 10 and 20 mut/Mb. Finally, there seems to be a noticeable overlap between MSI-H and TMB-H tumors, where the majority of MSI-H tumors are also TMB-H, but not vice-versa.
Our study reinforces the growing body of real-world evidence that TMB is a valid surrogate marker for MSI and can predict response to immune checkpoint blockade. We recognize that logistical challenges remain before wide-scale adoption of these biomarkers in clinical practice. Further research is necessary, especially focusing on the real-world application of NGS, histology-specific responses, and the importance of a specific cut-off for definition of TMB-H.
Conclusion
Our retrospective study of MSI-H and/or TMB-H patients highlights and reinforces the fact that TMB can act as a surrogate biomarker for MSI and that patients with either of these genomic statuses may benefit from immunotherapy. These results were regardless of tumor type and prior treatment regimen. Barriers exist to the timely implementation of NGS-based biomarkers and more data are needed in order to raise awareness about the clinical utility of TMB and MSI. Overall, we recommend that clinicians consider treating patients with either of these genomic events with an immunotherapy regimen regardless of their histology. TMB is an emerging tumor-agnostic biomarker for response to treatment with immunotherapy that may, in addition to MSI-H and PD-L1 staining, expand personalized cancer care in the era of immune-oncology. Further analysis or real-world data and generation of real-world evidence and larger prospective studies are warranted in multiple histologies, to better understand the MSI-H, TMB, and PD-L1 relationship.
Acknowledgments
Funding
None declared.
Disclosure
TAC is a cofounder of Gritstone Oncology and own stock. TC has received funding from Bristol Myers Squibb (BMS), AstraZeneca, Illumina, NysnoBio, Pfizer. VS reports grants from LOXO Oncology during the conduct of the study and Bayer outside the submitted work, and research funding/grant support for clinical trials (to his institution) from AbbVie, Agensys, Alfa-153 sigma, Altum, Amgen, Bayer, Berg Health, Blueprint, Boston Biomedical, Boston Pharmaceuticals, D3, Dragonfly Therapeutics, Exelixis, Fujifilm, Idera Pharma, Incyte, InhibRx, Loxo Oncology, Medimmune, MultiVir, NCI Cancer, Therapy Evaluation Program, National Comprehensive Cancer Network, Novartis, PharmaMar, Pfizer, Takeda, Turning Point Therapeutics, and University of Texas MD Anderson Cancer Center. RG reports research funding/grant support for clinical trials (to his institution) from Regeneron, BMS, Merck/EMD Serano, Amgen, Roche/Genentech, Philogen. Consulting/advisory board from Regeneron. Speaker for Deciphera. Currently employee of Merck. This manuscript and all work pertaining to it was done prior to employment at Merck. All other authors have declared no conflicts of interest.
References
- 1.Morrison C., Pabla S., Conroy J.M., et al. Predicting response to checkpoint inhibitors in melanoma beyond PD-L1 and mutational burden. J Immunother Cancer. 2018;6(1):32. doi: 10.1186/s40425-018-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ready N., Hellmann M.D., Awad M.M., et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol. 2019;37(12):992–1000. doi: 10.1200/JCO.18.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rizvi H., Sanchez-Vega F., La K., et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goodman A.M., Kato S., Bazhenova L., et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther. 2017;16(11):2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maymani H., Hess K., Groisberg R., et al. Predicting outcomes in patients with advanced non-small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer. 2018;120:137–141. doi: 10.1016/j.lungcan.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus L., Lemery S.J., Keegan P., Pazdur R. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25(13):3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 8.Panda A., Betigeri A., Subramanian K., et al. Identifying a clinically applicable mutational burden threshold as a potential biomarker of response to immune checkpoint therapy in solid tumors. JCO Precis Oncol. 2017;2017 doi: 10.1200/PO.17.00146. PO.17.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Snyder A., Makarov V., Merghoub T., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litchfield K., Reading J.L., Puttick C., et al. Meta-analysis of tumor- and T cell-intrinsic mechanisms of sensitization to checkpoint inhibition. Cell. 2021;184(3):596–614.e14. doi: 10.1016/j.cell.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristescu R., Mogg R., Ayers M., et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marabelle A., Fakih M., Lopez J., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 13.Subbiah V., Solit D.B., Chan T.A., Kurzrock R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) ≥10: a decision centered on empowering patients and their physicians. Ann Oncol. 2020;31(9):1115–1118. doi: 10.1016/j.annonc.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Prasad V., Addeo A. The FDA approval of pembrolizumab for patients with TMB >10 mut/Mb: was it a wise decision? No. Ann Oncol. 2020;31(9):1112–1114. doi: 10.1016/j.annonc.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Samstein R.M., Lee C.H., Shoushtari A.N., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51(2):202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valero C., Lee M., Hoen D., et al. Response rates to anti-PD-1 immunotherapy in microsatellite-stable solid tumors with 10 or more mutations per megabase. JAMA Oncol. 2021;7(5):739–743. doi: 10.1001/jamaoncol.2020.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Büttner R., Longshore J.W., López-Ríos F., et al. Implementing TMB measurement in clinical practice: considerations on assay requirements. ESMO Open. 2019;4(1):e000442. doi: 10.1136/esmoopen-2018-000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodman A.M., Sokol E.S., Frampton G.M., Lippman S.M., Kurzrock R. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer Immunol Res. 2019;7(10):1570–1573. doi: 10.1158/2326-6066.CIR-19-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parikh A.R., He Y., Hong T.S., et al. Analysis of DNA damage response gene alterations and tumor mutational burden across 17,486 tubular gastrointestinal carcinomas: implications for therapy. Oncologist. 2019;24(10):1340–1347. doi: 10.1634/theoncologist.2019-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrail D.J., Pilié P.G., Rashid N.U., et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661–672. doi: 10.1016/j.annonc.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jardim D.L., Goodman A., de Melo Gagliato D., Kurzrock R. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39(2):154–173. doi: 10.1016/j.ccell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan T.A., Yarchoan M., Jaffee E., et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019;30(1):44–56. doi: 10.1093/annonc/mdy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pepe F., Pisapia P., Gristina V., et al. Tumor mutational burden on cytological samples: a pilot study. Cancer Cytopathol. 2021;129(6):460–467. doi: 10.1002/cncy.22400. [DOI] [PubMed] [Google Scholar]
- 24.Schwaederle M., Parker B.A., Schwab R.B., et al. Precision oncology: the UC San Diego Moores Cancer Center PREDICT experience. Mol Cancer Ther. 2016;15(4):743–752. doi: 10.1158/1535-7163.MCT-15-0795. [DOI] [PubMed] [Google Scholar]
- 25.Carbone D.P., Reck M., Paz-Ares L., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larkin J., Minor D., D'Angelo S., et al. Overall survival in patients with advanced melanoma who received nivolumab versus investigator’s choice chemotherapy in CheckMate 037: a randomized, controlled, open-label phase III trial. J Clin Oncol. 2018;36(4):383–390. doi: 10.1200/JCO.2016.71.8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galvano A., Gristina V., Malapelle U., et al. The prognostic impact of tumor mutational burden (TMB) in the first-line management of advanced non-oncogene addicted non-small-cell lung cancer (NSCLC): a systematic review and meta-analysis of randomized controlled trials. ESMO Open. 2021;6(3):100124. doi: 10.1016/j.esmoop.2021.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Ami E., Barysauskas C.M., Solomon S., et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: results of a phase 2 study. Cancer. 2017;123(17):3285–3290. doi: 10.1002/cncr.30738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Groisberg R., Hong D.S., Behrang A., et al. Characteristics and outcomes of patients with advanced sarcoma enrolled in early phase immunotherapy trials. J Immunother Cancer. 2017;5(1):100. doi: 10.1186/s40425-017-0301-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu C.E., Yeh D.W., Pan Y.R., et al. Chromosomal instability may not be a predictor for immune checkpoint inhibitors from a comprehensive bioinformatics analysis. Life (Basel) 2020;10(11):276. doi: 10.3390/life10110276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salem M.E., Bodor J.N., Puccini A., et al. Relationship between MLH1, PMS2, MSH2 and MSH6 gene-specific alterations and tumor mutational burden in 1057 microsatellite instability-high solid tumors. Int J Cancer. 2020;147(10):2948–2956. doi: 10.1002/ijc.33115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salem M.E., Puccini A., Grothey A., et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018;16(5):805–812. doi: 10.1158/1541-7786.MCR-17-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]