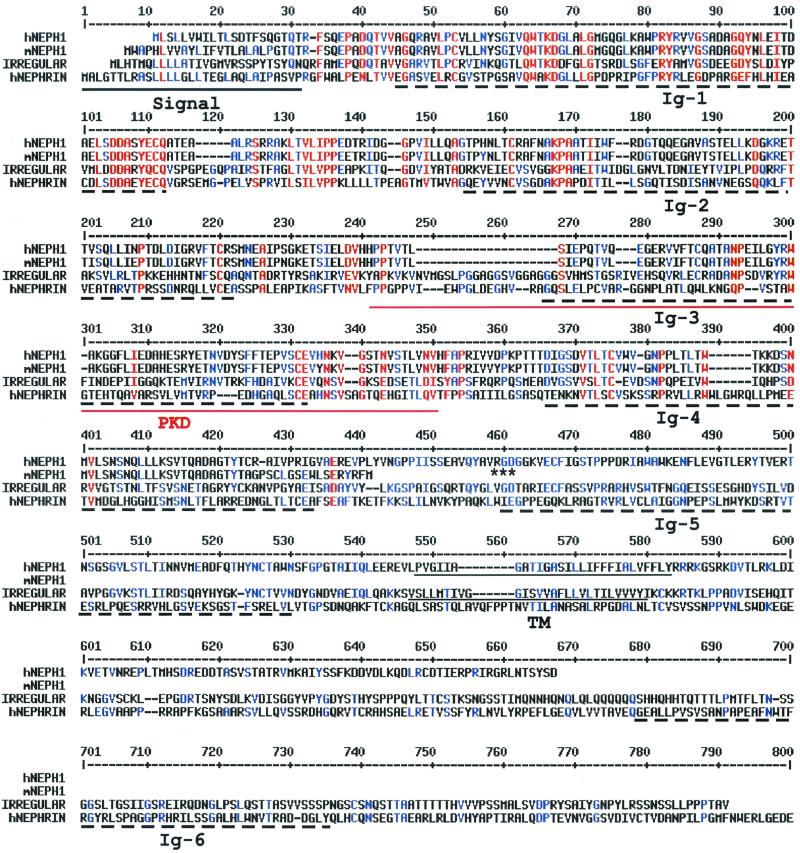
FIG. 1.
Alignment of NEPH1 with homologous proteins. The human NEPH1 (hNEPH1; GenBank accession no. AYO17369), a truncated mouse NEPH1 (mNEPH1; GenBank accession no. AYO17368), the D. melanogaster irregular chiasmata (IRREGULAR; GenBank accession no. L11040), and the human NEPHRIN (hNEPHRIN; GenBank accession no. AF190637) proteins were aligned using the MultiAlin version 5.4.1 program (http://www.toulouse.inra.ft/multalin). The signal and transmembrane (TM) domains in the hNEPH1, mNEPH1, and IRREGULAR proteins, identified by DNAStar Protean V. 4.0, are underlined. Ig-like domains (Ig) are marked by broken lines, and the PKD domain is doubly underlined; these domains were identified using the Pfam search engine (http://pfam.wustl.edu). The RGD sequence of NEPH1 is marked by asterisks. The hNEPHRIN protein sequence extends an additional 459 amino acid residues beyond what is shown here.