Figure 5. Activation of glial cells by TNFα reduced apoE secretion in APOE4 astrocytes, and increased apoE secretion in APOE4 microglia.
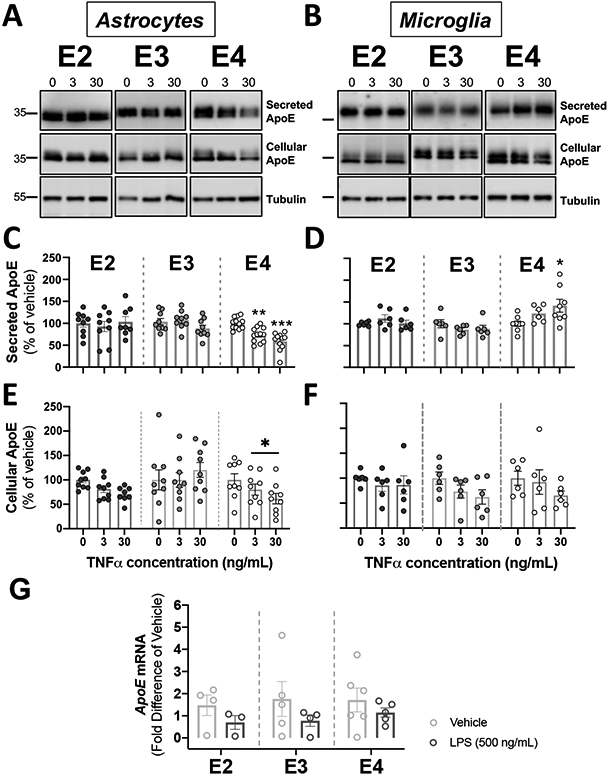
Glial cells derived from human APOE (E2, E3, E4) targeted-replacement mice were treated with 3-30 ng/mL of TNFα. Conditioned media and cells were collected at 24 hrs., and were analyzed by western blotting with antibodies against apoE. Tubulin was used as a loading control in cell lysates. (A-B) Representative immunoblots showing the changes in secreted and cellular apoE by astrocytes (A) and microglia (B) in response to TNFα. (C-D). Quantification of secreted apoE by western blots. (C) TNFα decreased apoE release in APOE4 astrocytes. Bar graphs represent the mean ± SEM (n=9-12, 3-4 experiments, run in triplicate). **p<0.01 ***p<0.005 compared to APOE4 untreated cultures. (D) TNFα increased apoE release in APOE4 microglia. Bar graphs represent the mean ± SEM (n=6-8, 3-4 experiments, run in duplicate). *p<0.05 compared to APOE4 untreated cultures. (E-F). Quantification of cellular apoE by western blots from cell lysates. (E) TNFα decreased cellular ApoE in APOE4 astrocytes. Bar graphs represent the mean ± SEM (n=7-9, 3 experiments, run in triplicate). *p<0.05 compared to APOE4 untreated cultures. (F) TNFα did not change cellular apoE in microglia. Bar graphs represent the mean ± SEM (n=6, 3 experiments, run in duplicate). (C-F) A one-way ANOVA was used to assess outcome measures from pharmacological manipulation, setting vehicles at 100%. (G) TNFα did not change APOE mRNA in astrocytes. APOE mRNA expression was expressed as fold difference from vehicle. Bar graphs represent the mean ± SEM (n=3-6, 3 experiments, run in duplicate). A two-tailed Student T-test was used to assess outcome measures from pharmacological manipulation, setting vehicle treatment as control.
