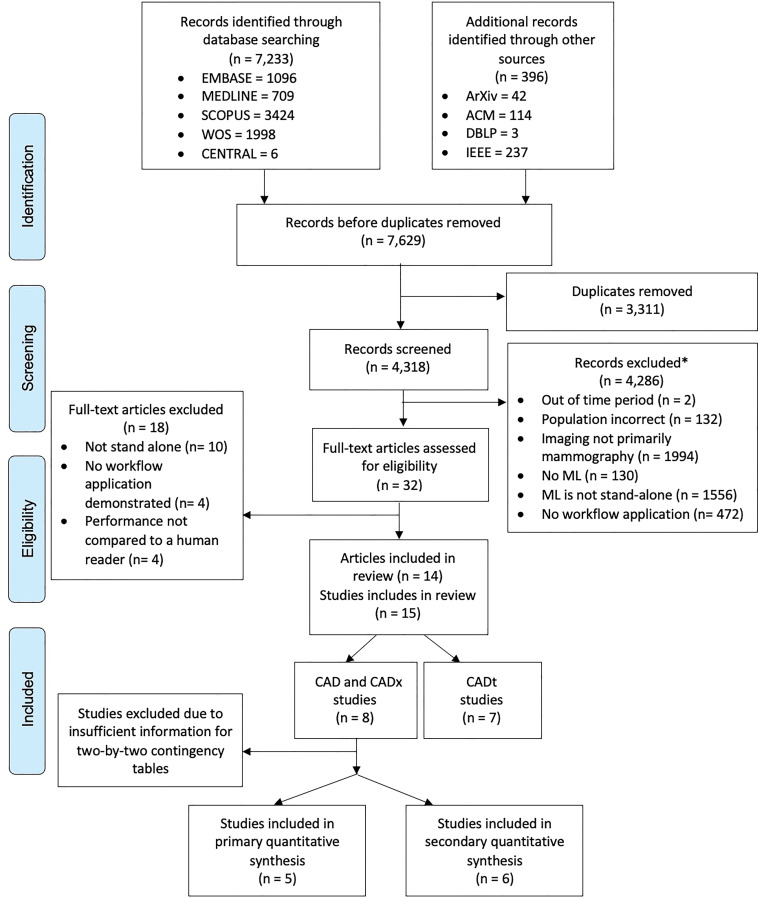
Figure 2:
Flowchart of Preferred Reporting Items for Systematic Review and Meta-Analysis for Diagnostic Test Accuracy for studies included in identification, de-duplication, screening, and data-extraction stages of review. ACM = Association for Computing Machinery, CAD = computer-aided detection, CADt = computer-aided triage, CADx = computer-aided diagnosis, IEEE = Institute of Electrical and Electronics Engineers, ML = machine learning, WOS = Web of Science. * = Studies could have been excluded for multiple reasons.