Table 1.
Optimization of HBD catalyst structure and reaction conditionsa
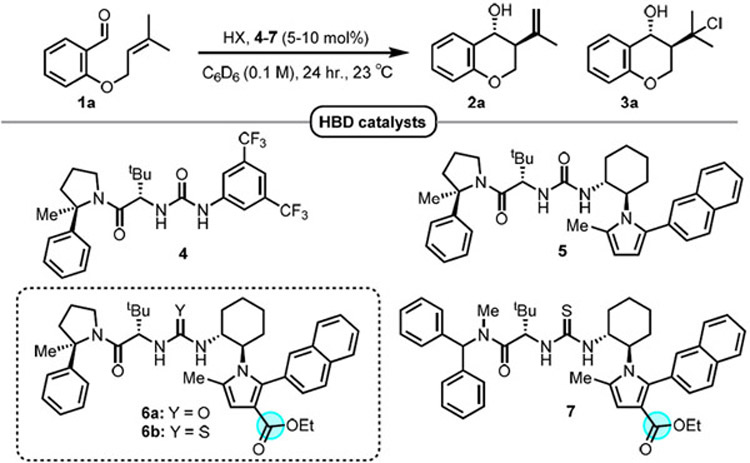
| ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | HX (mol%) |
HBD (mol%) |
conversion (%)b |
yield (%)b |
d.r. 2ab | 2a : 3ab | ee 2a (%)c |
ee 3a (%)c |
| 1 | — | 4 (10) | n.r. | n.r. | — | — | — | — |
| 2 | HCl (8) | 4 (10) | 33 | 28 | 10 : 1 | 3.5 : 1 | 76 | 74 |
| 3 | MsOH (8) | 4 (10) | 100 | trace | — | — | — | — |
| 4 | TsOH H2O (8) | 4 (10) | 87 | 30 | 4 : 1 | — | 31 | — |
| 5 | BzOH (8) | 4 (10) | n.r. | n.r. | — | — | — | — |
| 6 | HOP(O)(OPh)2 (8) | 4 (10) | n.r. | n.r. | — | — | — | — |
| 7 | HCl (8) | 5 (10) | 41 | 32 | 22 : 1 | 6.5 : 1 | 89 | 77 |
| 8 | HCl (8) | 6a (10) | 45 | 44 | 23 : 1 | 4.4 : 1 | 96 | 95 |
| 9 | HCl (8) | 6b (10) | 34 | 32 | 49 : 1 | 3.0 : 1 | 98 | 98 |
| 10 | HCl (8) | 7 (10) | 35 | 25 | 12 : 1 | 2.4 : 1 | 87 | 89 |
| 11 | HCl (30) | 6b (10) | 96 | 94 | > 50 : 1 | 3.1 : 1 | 96 | 98 |
| 12 | HCl (30) | 6b (5) | 88 | 85 | 30 : 1 | 3.1 : 1 | 92 | 94 |
| 13 | AcCl + EtOH (30) | 6b (5) | 93 | 91 | 38 : 1 | 3.0 : 1 | 95 | 97 |
| 14d | AcCl + EtOH (30) | 6b (5) | — | 82 | 36 : 1 | — | 95 | — |
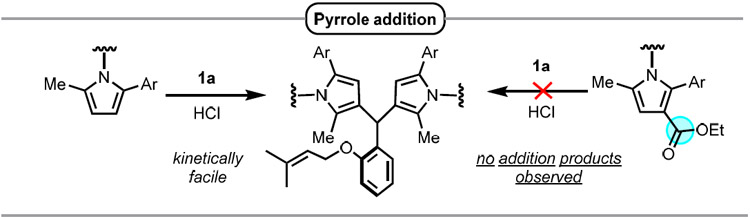
| ||||||||
Conducted using 0.08 mmol of 1a with Brønsted acid cocatalysts delivered as solutions in Et2O.
Determined from crude reaction mixtures using 1H NMR spectroscopy with mesitylene internal standard. Yield refers to the combined yield of products trans/cis 2a and 3a except for entry 14.
Determined by GC analysis using a chiral stationary phase. n.r. = no reaction.
Reaction was quenched by the addition of 60 mol% KHMDS.
