Fig. 6|. Proposed mechanism of activation of Arp2/3 complex by Dip1.
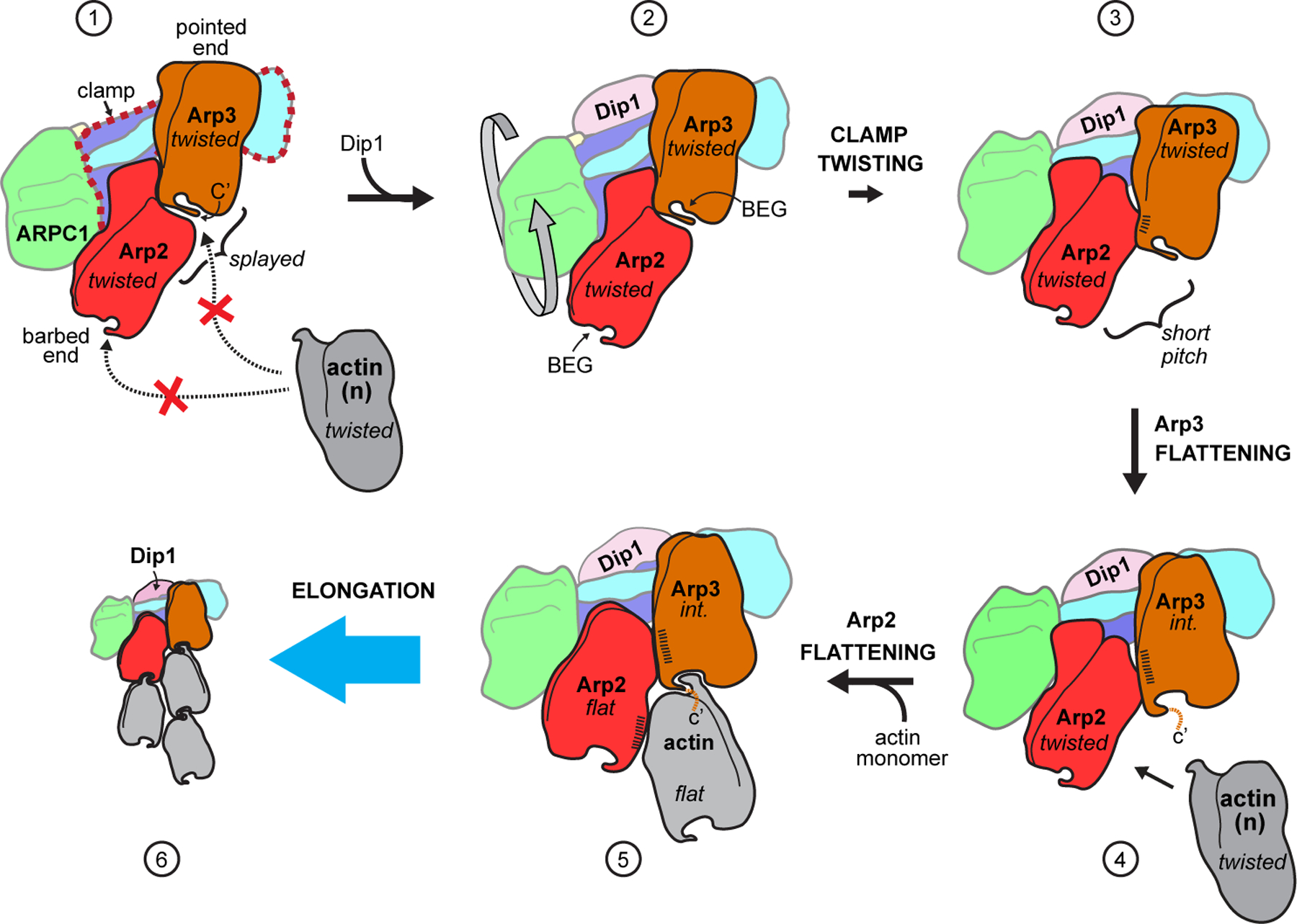
In state 1, Arp2/3 complex is in an inactive state with the barbed end of Arp3 blocked by Arp2 and the barbed end grooves (labeled BEG in state 2) are not properly arranged for interaction with actin. Dip1 binds to the clamp (red dashed outline in state 1) to stimulate clamp twisting and movement of Arp2 into the short pitch conformation (states 2 to 3). Arp2 in the short pitch conformation triggers partial flattening of Arp3, which opens up the Arp3 barbed end groove for optimal long pitch interactions with actin n (states 3 to 4). Steps 4 and 5 show actin monomer n flattens and stimulates flattening of Arp2 upon binding to the complex, however, it is possible that formation of the nucleus requires binding of additional actin monomers to the nascent filament. Dip1 binds tightly to activated Arp2/3 complex on the pointed end of the actin filament17 (state 6), unlike WASP, which must be released for nucleation to proceed. Conformational states are indicated in italicized text; int.: intermediate (partially flattened) state of Arp3. Hatching indicates relative surface area buried at short pitch interface. For clarity, ARPC3 is not depicted in this cartoon.
